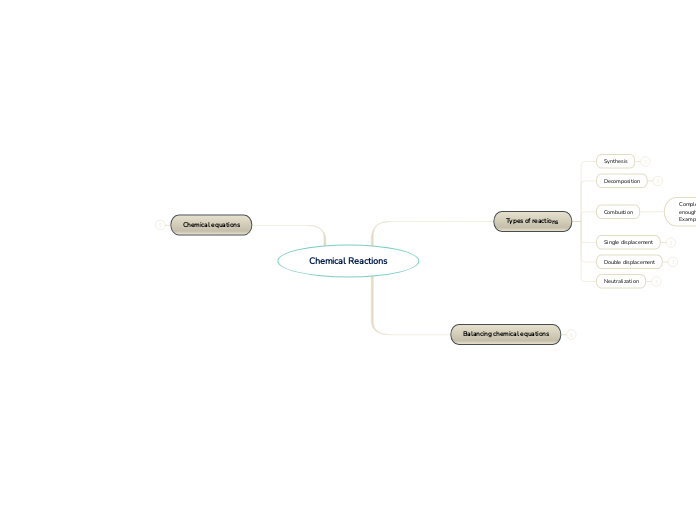
Synthesis
Decomposition
Combustion
Complete combustion: Complete combustion only occurs when there is enough oxygen present.
Example: C3H8 + 5 O2 → 3 CO2 + 4 H2O + Heat
Incomplete combution: Incomplete combustion takes place when there is not enough oxygen to allow the fuel to react completely and burn in order to produce carbon dioxide and water.
Example: 2 C3H8 + 9 O2 → 4 CO2 + 2 CO + 8 H2O + Heat
Single displacement
Double displacement
Neutralization