chemistry
acids and base

indicators

levels

properties

composition
H
OH
Types of reactions

balancing (Law of conservation of mass)
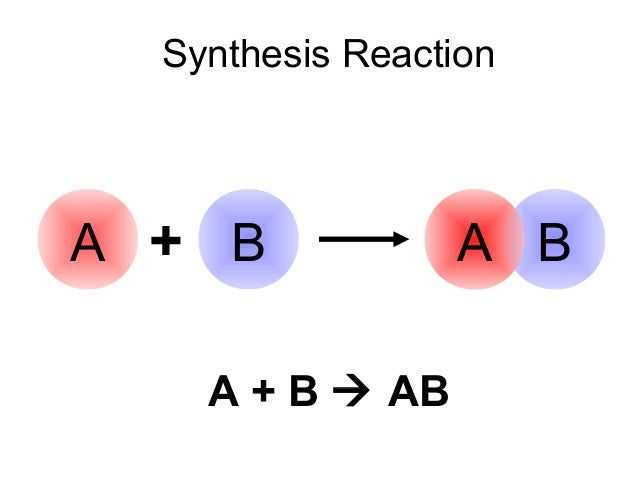
synthesis

decomposition
/glowing-flasks-56a12dd83df78cf772682dd6.jpg)
single displacement

Double displacement
Maatters

elements

compounds
pure substances
Bonding

molecular

counting atoms

example H2O

Subtopic
models
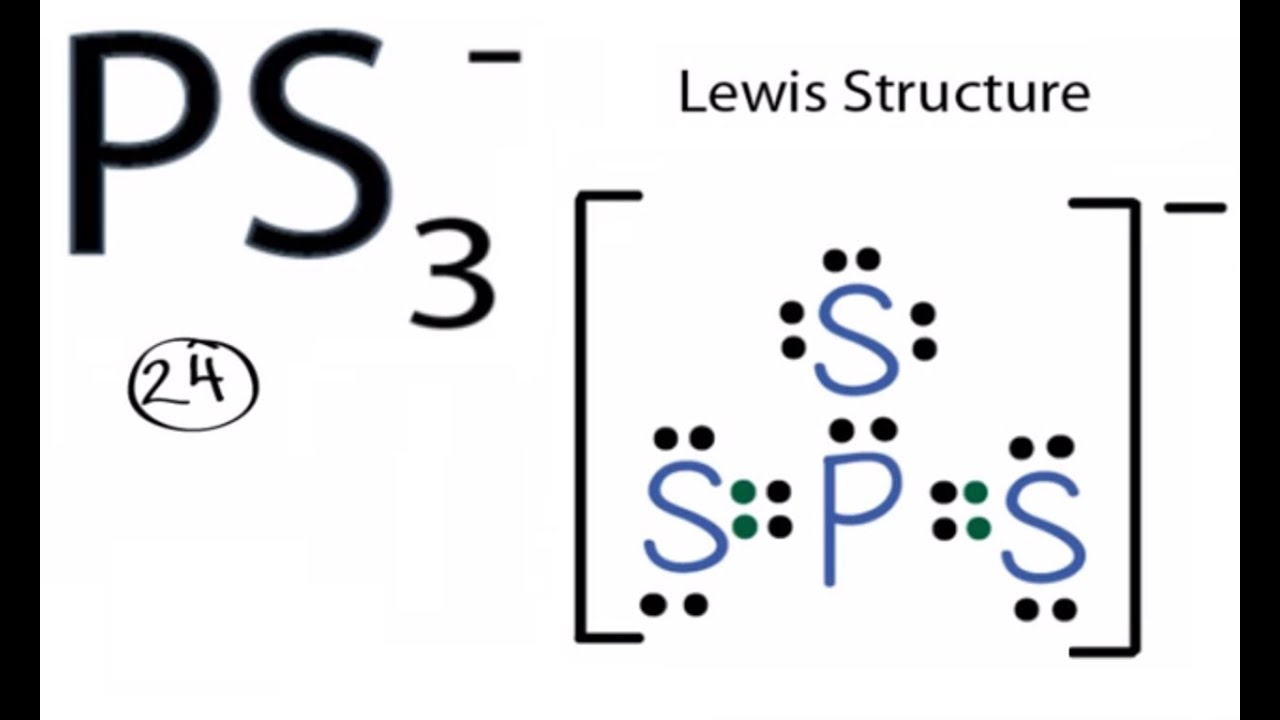
Lewis diagram

Bohr Rutherford

ionic
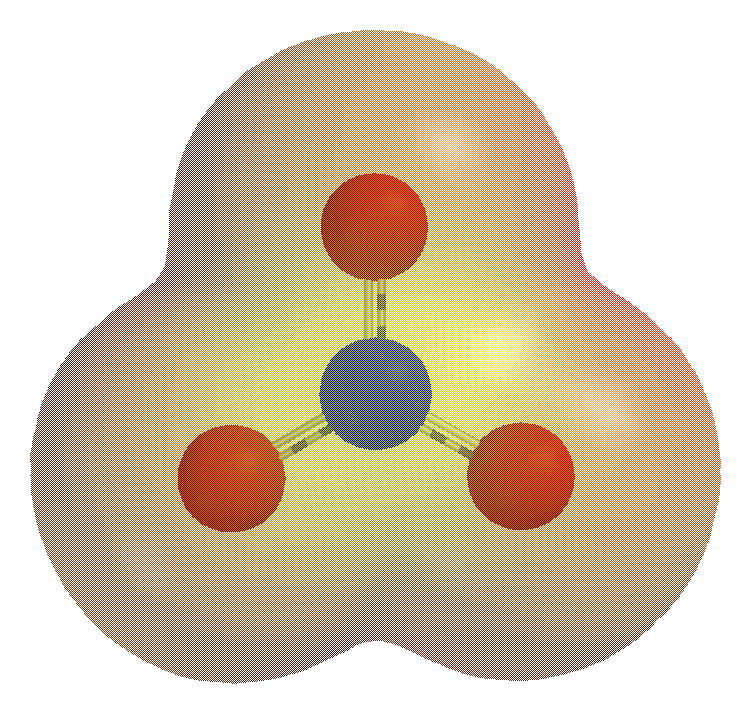
ions

poly atomic ions