Metabolism
All the chemical reactions that change of transform matter and energy in cells. These rxns occur in step-by-step sequences called "metabolic pathways"Important Terms to keep in mind:Activation energy: NRG required to initiate a reactionA catalyst (such as enzymes) lower the activation energy
Thermodynamics and Metabolism
ATP
Adenosine TriPhosphate- referred to as the NRG of the cell.Is composed of:Nitrogenous base AdenineRibose sugarThree Phosphate Groups
ATP/ADP cycle
When ATP is hydrolyzed (broken down) it releases energyAll phosphates are negative so the groups strain away from each otherWhen 1 bond is broken to ADP - energy is releasedHighly exergonic reaction (releases energy)
Exergonic
NRG for cellular reactions
Endergonic
NRG for cellular processes
Electron Carriers
Electrons carriers: compounds that pick up electrons from energy-rich compounds and donate them to low-energy compoundsElectron carriers are recycled:NAD+ (oxidized) gains electrons and becomes NADH- (reduced from) FAD (oxidized) gains electrons and becomes FADH2 (reduced from) Ex. are like Robin hood in the sense that they pick up electrons and give them to electron poor molecules *NADH is a coenzyme that can help transfer electrons
NADH
reduced (gains electrons)
NAD+^
Oxidized (loses electrons)
FADH2
Reduced form (gains electrons)
FAD
Oxidized (loses electrons)
Coupled Rxns
Use NRG from hydrolysis as input for the build of ATP (NRG generated from one rxn is used to drive second rxn) Redox rxns are couples rxns Electrons that pass from one atom to another and carry energy with themTherefore, reduced molecules (gained electrons) have higher energy levels compared to oxidized molecules
Main topic
Types of Energy
Energy: the capacity to do work (to change or move matter against an opposing force like friction or gravity).
Kinetic
NRG in motion (moving objects perform work by casing other matter to move)
Electrical
Electro-magnetic
Potential
Stored NRG (NRG that us available but not yet released)
Elastic
Gravitational
Chemical
Bond
NRG required to break of form a chemical bond
In labs
Energy released is usually thermal energy (heat)
In living organisms
Thermal energyMovement of compounds across cell membranesContraction of a muscleEmission of light (certain organisms - jellyfish)
Exothermic
Exothermic: A reaction or process accompanied by the released of energy in the form of heat. Is a catabolic rxn.Biology ex. Cellular respiration When a chemical bond forms between two atoms, energy is releasedEx. Zn+S --> ZnS (NRG between two atoms used to make a compound releases NRG making it exothermic)ΔH= H of products- H of reactants*When reactants have more energy than the products, then it is exothermic
Endothermic
Endothermic: A reaction or process accompanied by or requiring the absorption of energy in the form of heat. Is an anabolic rxn. Biology ex. Photosynthesis (absorption of light NRG)Amount of energy to break that bond is the same amount of energy that was releasedEx. ZnS --> Zn + S (Bonds absorbed more energy so the compound can decompose making it endothermic)ΔH= H of products- H of reactants*When products has more energy than the reactants it is endothermic
Subtopic
Laws of Thermodynamics
Thermodynamics: The science that studies the transfer and transformation of thermal energy
1st Law
Def: Energy cannot be created or destroyed, but it can be transformed from one type into another and transferred from one object to anotherAs a process progresses, the amount of NRG is maintained (never destroyed just transferred) "Law of conservation of NRG" (total NRG in the universe remains constant)
Heat Example
Ex. when you hold a coffee cup, the heat from the cup is exerted onto your hand. The thermal energy is still there but it was transferred from the cup onto the hand. Energy isn't destroyed it is transferred into the surroundings out of the open system.
2nd Law
Def: During any process, the universe tends toward disorder. Since disorder is more likely to happen as opposed to order. Every time an energy transfer happens, some amount of useful energy will move from the useful to the useless category.Any process, such as a chemical reaction or set of connected reactions, will proceed in a direction that increases the overall entropy of the universe.Heat energy not being used will go toward increasing randomness (increasing entropy) Law pertains to closed systems (as organisms are highly ordered) The high degree of organization of living things is maintained by a constant input of energyIs offset by an increase in the entropy of the surroundings. (input being carbs and released through heat).
Entropy
Entropy: The degree of randomness or disorder in a system is called its entropy. (the measure)Entropy is continuously increasing (from the disorder from the universe) *With more randomness, there's more disorder (more entropy)
Entropy in biological systems
walking contracts muscles and uses energy derived from macromolecules (glucose) chemical energy from food is being converted to kinetic large fraction of energy from fuel is transformed into heat some stays within the body, but some exits into the surroundings further increasing entropy
System
In biology: A biological system is a group of organs working together to perform a common function. Example: organisms, groups of cells, or a set of substrates/product*Connection to chemistry- a thermal system is involved in the storage and transfer of heat. (similar to how organs function in the body)
Open
Exchange of matter and energy
Closed
Exchange of energy
Surroundings
Surroundings: Everything in the universe outside of the system
Catabolism: releases NRG cuz it breaks down glucose forming glucose for ATP, NADH, NADPH
Anabolism: Absorbs NRG because it builds it up for ADP, NAD and NADP
Summary of Kinetic NRG

Visual Aid
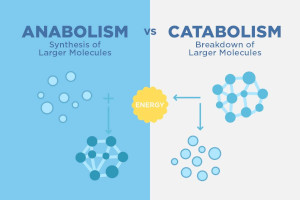
Anabolism and Catabolism
Anabolsim is the process of using NRG to build large molecules Catabolsim is the process of breaking down compounds into smaller molecules to release NRG

/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
Visual aid

visual aid
/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)