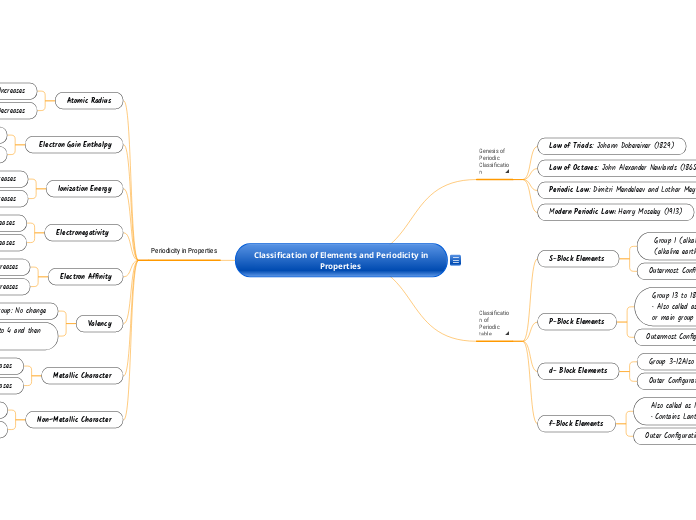
Classification of Elements and Periodicity in Properties
Genesis of Periodic Classification^
Law of Triads: Johann Dobereiner (1829)
Law of Octaves: John Alexander Newlands (1865)
Periodic Law: Dimitri Mendeleev and Lothar Meyer.
Modern Periodic Law: Henry Moseley (1913)
Classification of Periodic table^
S-Block Elements
Group 1 (alkali metals) and Group 2
(alkaline earth metals)
Outermost Configuration is
P-Block Elements
Group 13 to 18.
• Also called as representatives
or main group elements
Outermost Configuration varies to
d- Block Elements
Group 3-12Also called as Transition elements
Outer Configuration
f-Block Elements
Also called as Inner Transition Elements.
• Contains Lanthanoids and Actinoids
Outer Configuration is
Periodicity in Properties
Atomic Radius
Group: Increases
Period: Decreases
Electron Gain Enthalpy
Group: becomes less negative
Period: becomes more negative
Ionization Energy
Group: Decreases
Period: Increases
Electronegativity
Group: Decreases
Period: Increases
Electron Affinity
Group: Decreases
Period: Increases
Valency
Group: No change
Period: Increase from 1 to 4 and then
decrease from 4 to 0.
Metallic Character
Group: Increases
Period: Decreases
Non–Metallic Character
Group: Decreases
Period: Increases