Properties of Matter
Physical Properties
These describe the properties of a substance through the five senses: Hearing, smelling, seeing, touching and hearing.
Lustre
Defines how well a substance reflects light of its surface.
This chandelier is very lustrous, meaning
it reflects light very well off of its surface.
Solubility
Defines the ability of a substance(the solute) to dissolve when put in a solvent.

When the sugar(solute) is put
in water(solvent) it dissolves
and creates a new mixture
(a solution). This means sugar
is highly soluble.
Viscosity
Defines how fast or slow a liquid flows when being poured.

Honey flows very slowly when its poured, meaning
it's highly viscous.
Electrical conductivity
Defines how well a substance transfers electricity through it.

Gold is used in many
devices like phones
because gold has high
conductivity(transfers
electricity well)
Brittleness
If a substance is hard but can be easily broken into several pieces, it's considered brittle.

Glass is a hard substance, but
it breaks with a small amount
of force applied to it. This means
the glass is very brittle.
Hardness
This refers to the ability
of a substance to resist
force and keep its form.

Diamonds are extremely hard,
which is why they're used for
valuable things like marriage
rings.
Matter: What makes up everything in the universe. Matter takes up space wherever you go, and can have both physical and chemical properties. Read on to learn more!
Chemical Properties
Chemical properties describe the ability
of a substance to create another substance
Reactivity
The measurement of how much of a chemical change a substance undergoes when mixed with another substance.
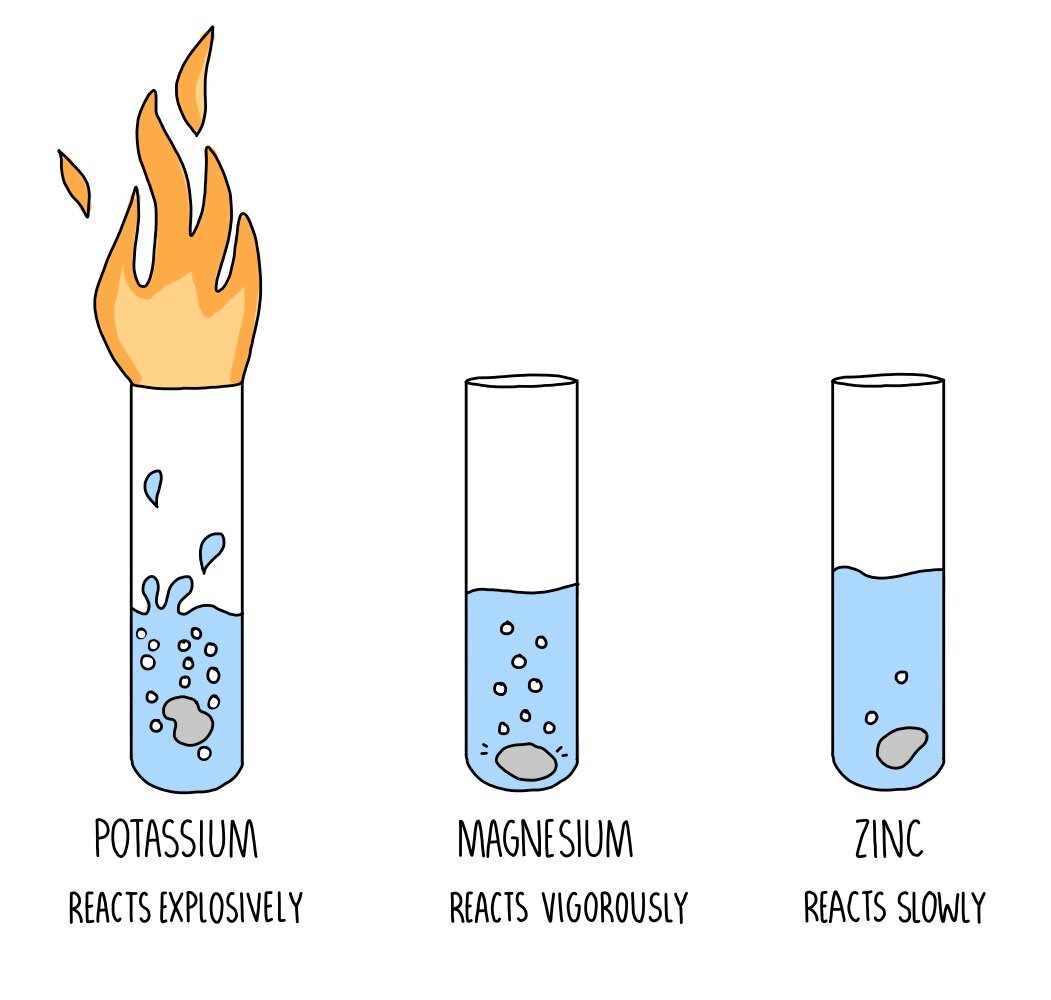
From this diagram, we can see that the
potassium had the most extreme reaction
to being put in water, starting a fire. This
means that potassium is highly reactive
in water.
Combustion
The ability of a substance to create heat
when exposed to oxygen.

Pouring gasoline in a fire will
increase the fire, because
gasoline becomes fire when
the air is hot(only above -45
degrees!).