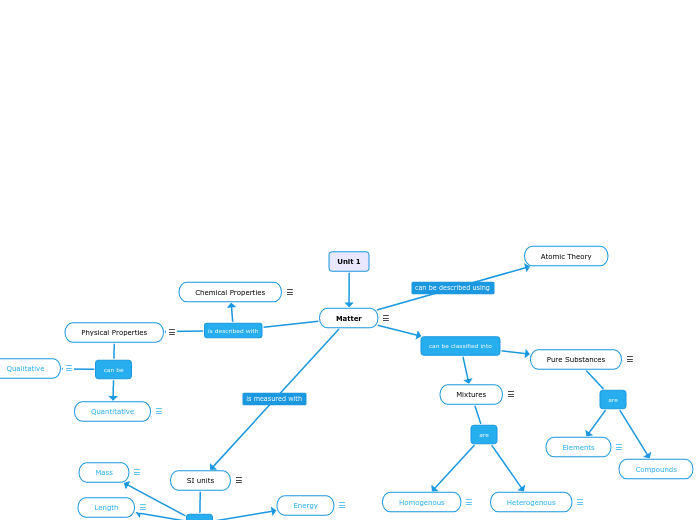
anything that has mass or volumecan be in solid, liquid, or gas states
is described with
Physical Properties
"a property that you can observe without changing one kind of matter into something new"
can be
Qualitative
"You can describe them in words, but you cannot measure them or express them numerically."physical statecolourodourcrystal shapemalleabilityductilityhardnessbrittleness
Quantitative
"can be measured and expressed numerically"melting pointboiling pointdensitysolubilityelectrical conductivitythermal conductivity
Chemical Properties
"a property that you can observe when one kind of matter is converted into a different kind of matter"reactivity with waterreactivity with airreactivity with pure oxygenreactivity with acidsreactivity with pure substancescombustibility (flammability)toxicitydecomposition
SI units
a standard system of measurement called the International System of Units which allows scientists anywhere in the world to describe matter in the same quantitative language
i.e.
Mass
the amount of matter in an objectmeasured by:kilogram (kg)gram (g)milligram (mg)with a balance
Length
the distance between two pointsmeasured bymetre (m)centimetre (cm)millimetre (mm)with a ruler
Mole
the amount of a substanceunit: mole (mol)calculated not measured
Temperature
the hotness or coldness of a substancemeasured bykelvin (K)degrees Celsius ( ̊C)with a thermometer
Energy
the capacity to do work (to move matter)unit: joule (J)calculated, not measured
Density
the mass per unit of volume of a substancemeasured bykilograms per cubic metre (kg/m3)grams per cubic centimetre (g/cm3)calculated or measured
Volume
the amount of space that an object occupiesmeasured by cubic metre (m3)cubic centimetre (cm3)litre (L)millilitre (mL)with a beaker, graduated cylinder, or pipette; may also be calculated
can be classified into
Mixtures
physical combinations of matter in which each component retains its identity
are
Homogenous
components are blended so that it looks like a single substance.
Heterogenous
all components are visible
Pure Substances
matter that has a definite composition
are
Elements
matter that cannot be decomposed into simpler substances
Compounds
matter in which two or more elements are chemically combined
Atomic Theory