Bio Concept Maps
Topic 1: Cell Structures and Functions
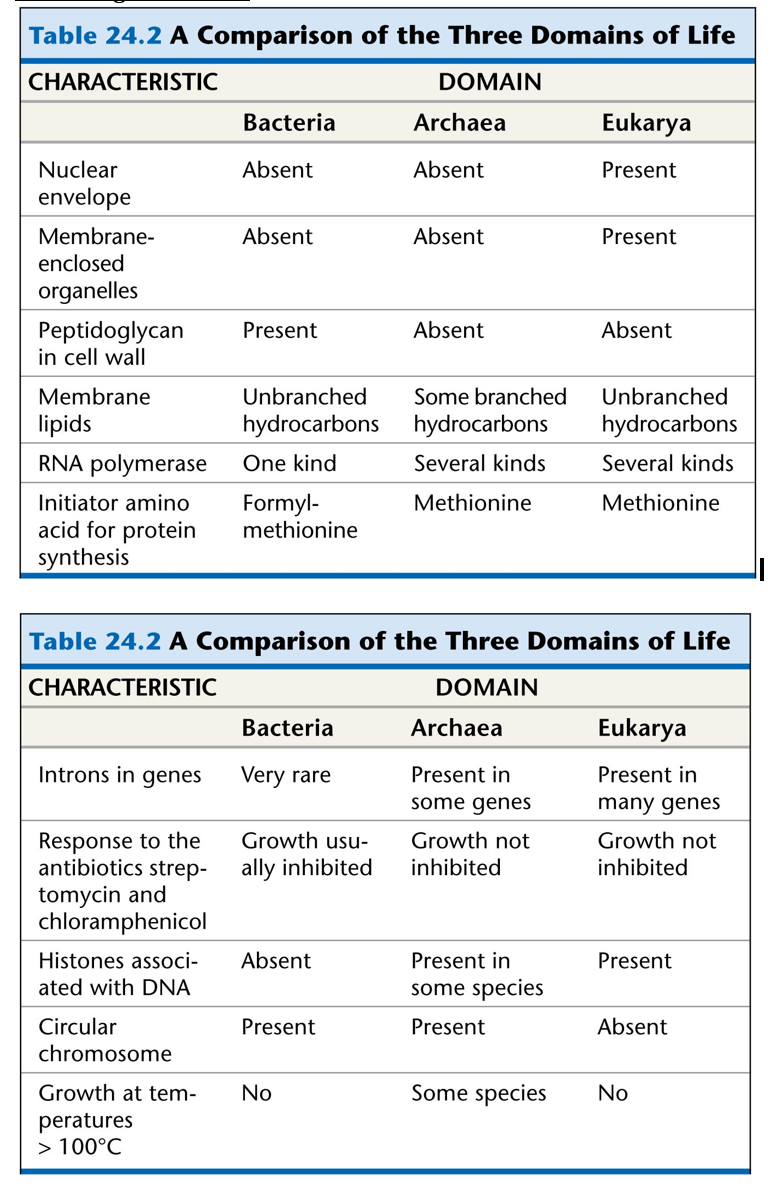
The Three Domains of Life:
Prokarya
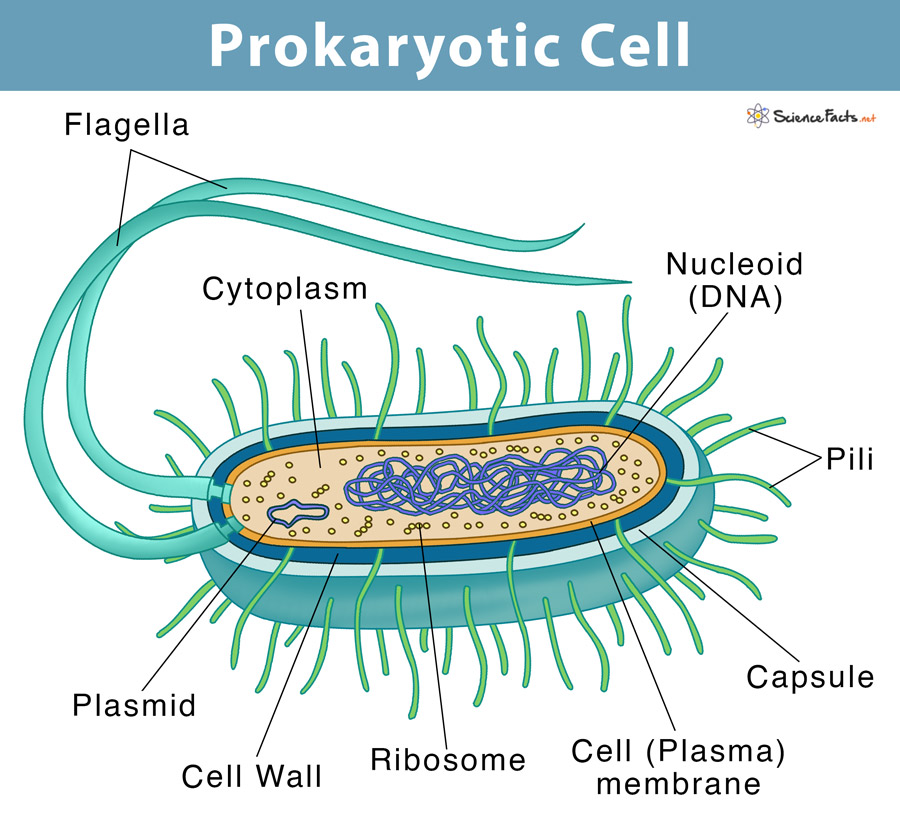
Prokaryotic Cells
Structure of Cell
Organelles of a Prokaryotic Cell:
Bacteria
KEY: Bold - Bacterial ChromosomeUnderline - Found in both bacterial chromosomes and 'a thin section through the bacterium Corynebacterium Diphtheriae'Fimbrae: attactment structures on the surface of some prokaryotes (not visible on TEM) *TEM is transmission electron microscopeNucleotide: region where the cell's DNA is located (not enclosed by a membrane)Ribosomes: complexes that synthesize proteinsPlasma Membrane: membrane enclosing the cytoplasmCell Wall: rigid structure outside the plasma membraneGlycocalyx: outer coating of many prokaryotes, consisting of a capsule or a slime layerFlagella: locomotion organelles of some prokaryotesComponents of a Typical Bacterial Cell: Plasma Membrane: selectively permeable barrier, mechanical boundary of cell, nutrient and waste transport, location of many metabolic processes (respiration, photosynthesis) detection of environmental cues for chemtaxisGas Vacuole: Buyancy for floating in aquatic environmentsRibosomes: protein synthesisInclusion bodies: storage of carbon, phosphate, and other substancesNucleoid: localization of genetic material (DNA)Periplasmic Space: Contains hydrolytic enzymes and binding proteins for nutrient processing and uptakeCell Wall: gives bacteria shape and protection from lysis in dilute solutionsCapsules and Slime Layers: resistance to phagocytosis, adherence to surfacesFimbriae and Pili: attachment to surfaces, bacterial matingFlagella: movementEndospore: Survival under harsh environmental conditions*Not present in all prokaryotes
Archaea
Components of an Archaea CellLack a defined nucleus which puts them into the prokaryotic categoryUnicellularReproduce AsexuallyPlasmidsInclusionsFlagellaPiliSometimes may contain Capsules and Slime Layers but can be very rareKnown for being extremophiles living in areas of extreme conditions such as high in acid, salt, or heatLinkage between Phospholipid head and side chain is in the form of L-isomeric formPlasma Membrane is found in the form of monolayersCell wall in archaea made to fit the environment of the organism. In some cases may be no cell wallFlagella is different from bacteria in that it is powered by ATPIncludes Cell Wall but is lacking peptidoglycan which is a distinguishing feature between bacteria and archaea. Instead, they contain pseudomureinHamus- long helical tube with three hooks that allow the cell to attach to other cells and to surfaces which encourages the formation of a communityCannulae- hollow tube structures that connect cells after divisionArchaeal ribosomes are different in shape from any other ribosome and contain proteins unique to archaeaContain ether bonds linking fatty acids to molecules of glycerolRNA polymerase of archaea contain more than eight polypeptidestRNA has unmodified methionineMetabolism differs from bacteria in that there are various types such as dentrification, nitrogen fixation, hyperthermophilic growth, and chemolithotrophy.
Major Nutritional Modes of Prokaryotes:
Autotroph: Photoautotroph: Energy Source: LightCarbon Source: CO2, HCO3^-, or related compoundsTypes of Organisms: Photosynthetic prokaryotes (ex: cyanobacteria), plants, certain protists (ex: algae)Chemoautotroph: Energy Source: Inorganic chemicals (ex: H2S, NH3, or Fe^2+) Carbon Source: CO2, HCO3^-, or related compoundsTypes of Organisms: Unique to certain prokaryotes (ex: sulfolobus)Heterotroph:Photoheterotroph: Energy Source: LightCarbon Source: Organic CompoundsTypes of Organisms: Unique to certain aquatic and salt-loving prokaryotes (ex: Rhodobacter, Chloroflexus) Chemoheterotroph: Energy Source: Organic CompoundsCarbon Source: Organic CompoundsTypes of Organisms: Many prokaryotes (ex: Clostridium), protists, fungi, animals, and some plants
Cell Membrane
All prokaryotic cells are surrounded by a plasma membrane, but they do lack membrane-bound organelles. Bacteria cells contain a fatty acidArchaea cells contain a Hydrocarbon
Eukarya

Eukaryotic Cells
Structure of Cell
Organelles of Eurkaryotic Cells:
Animal Cells:
Flagellum: motility structure present in some animal cells, composed of a cluster of microtubles within an extension of the plasma membraneCentrosome: region where the cell's microtubules are initiated; contains a pair of centriolesPerixisome: organelle with various specialized metabolic functions, produces hydrogen peroxide as a by-product and then converts it to waterMitochondrion: organelle where cellular respiration occurs and most ATP is generatedLysosome: digestive organelle where macromolecules are hydrolyzedGolgi apparatus: organelle active in synthesis, modification, sorting, and secretion of cell productsRibosomes: complexes that make proteins; free in cytosol or bound to rough ER or nuclear envelopePlasma membrane: membrane enclosing the cellCytoskeleton: reinforces cell’s shape; functions in cell movement; components are made of protein (includes: microfilaments, intermediate filaments, and microtubules)Endoplasmic Reticulum (ER): network of membranous sacs and tubes; active in membrane synthesis and other synthetic and metabolic processes; has rough (ribosome-studded) and smooth regions (includes rough ER and smooth ER) Nucleus:Nuclear envelope: double membrane enclosing the nucleus; perforated by pores; continuous with ERNucleolus: nonmembranous structure involved in production of ribosomes; a nucleus has one or more nucleoliChromatin: material consisting of DNA and proteins; visible in a dividing cell as individual condensed chromosomes
Plant Cells:
Nucleus: includes nuclear envelope, nucleolus, chromatinCytoskeleton: includes microfilamentsGolgi ApparatusMitochondriaRibosomesSmooth and Rough Endoplasmic ReticulumPeroxisomeCell wall: outer layer that maintains cell’s shape and protects cell from mechanical damage; made of cellulose, other polysaccharides, and proteinPlasmodesmata: cytoplasmic channels through cell walls that connect the cytoplasms of adjacent cellsChloroplast: photosynthetic organelle; converts energy of sunlight to chemical energy stored in sugar moleculesCentral vacuole: prominent organelle in older plant cells; functions include storage, breakdown of waste products, and hydrolysis of macromolecules; enlargement of the vacuole is a major mechanism of plant growth*Italicized is specific to Plant Cells

Extra:

Subtopic
Cell Membrane
The cell membrane separates the inside of the cell from what is outside. It controls what can go inside and outside the cell. It also helps with the transportation for the molecules.
Bulk Transport
Mode of transport across the membrane. This occurs when there are small particles crossing across the cell without using the membrane.
Endocytosis
Occurs when small particles enter the cell. Using the transportation bulk transport.
Engulfing of substances
Phagocytosis
Small food or large particles go into the cell and create a vacuole to stay in there.
"Eating"
By putting food in a vacuole
Pinocytosis
This occurs when liquids and small particles go into the cell. They create vacuoles to stay in the cell.
"Drinking"
By placing molecules into a vesicle
Receptor-Mediated Endocytosis
This occurs when the receptor proteins in the surface see a molecule they want from the outside. They create a coated vesicle to keep the molecule inside.
Ligand required to
to bind to receptor
Molecules are places in a COATED vesicle
Exocytosis
Occurs when small particles exit the cell as bulk transport.
Excrement of substance
Active Transport
Active transport is a mode of transportation across the membrane that uses energy. The molecules go from a low concentration to a high concentration. They move across against the concentration gradient.
Moving substance against
concentration gradient
Required ATP
Passive Transport
Transportation across the membrane with no use of energy.
Diffusion
This is a mode of passive transport. The molecules cross the membrane from a high concentration to low concentration to create a equilibrium.
Movement of small nonpolar substance
From High Concentration
To Low Concentration
Osmosis
This is a mode of passive transport. The movement of water molecules from a low concentration to a low concentration. For example sugar cant cross the membrane so it needs water to dissolve and cross correctly.
Which move water
From Low Concentration
To High Concentration
Facilitated Diffusion
This is a mode of passive transport. This is used when certain molecules can't pass the membrane. When this happens proteins can be used. The protein will have a channel that will allow certain solutes to cross the membrane.
Requires a PROTEIN
To move large/polar molecules
Common Components
of Cells
DNA
Ribosome
Cytoplasm
Mitochondrion
Macro Chemistry
Carbs
Forms Glycosidic Linkages
Polymerizes through dehydration
Provides ENERGY for the cell
Lipids
Forms Ester Linkages
Between Glycerol and Fatty Acid
Polymerizes through dehydration
Crucial to Phospholipid bilayers in the cell
Cell Exterior Membrane
Membrane bound organelles
Can come in two forms
Saturated
Superimposes into a unit structure
If superimposed, the membrane will be less fluid.
Which make diffusion harder
No Double bonded Carbons
Unsaturated
Double Bonded Carbons
Not easily superimposable
If superimposed, the membrane will be more fluid.
Which makes diffusion easier
Proteins
Form Peptide Bond
In Primary Structure
Polymerizes through dehydration
Form Hydrogen Bonds
In Secondary Structure
Form Disulfide Bonds
Ionic Bonds
Ion-Dipole Interactions
Hydrophobic Interactions
Hydrogen Bonds
In Tertiary and Quaternary
Responsible for Functions in the Cell
Transport
Enzymatic Activity
Which breaks down molecules
This is Catabolic
Builds up molecules to more
complex structure
This is Anabolic
Signal Transduction
Explains how a signal (ligand)
leads to a response
Cell-Cell recognition
Nucleic Acids
Forms Phosphodiester Linkages
Between C3 and C5
Polymerizes through dehydration
Forms Hydrogen bonds
Between Nitrogenous Bases
Which Consist of Purines and Pyrimidines
Purines: A/G
Pyrimidines: U/T/C
Carries genetic material of the cell
Like DNA
And RNA
Responsible for Proteins Synthesis
Through Transcription of DNA to mRNA
Then translates mRNA to a functional protein
Topic 3: DNA structure, replication,
expression and regulation:
DNA Structure and Replication
Structure
Fredrick Griffith (1928)
In 1928, Fredrick Griffith tried developing a vaccine against pneumonia. He injected mice with these cells using two strains of the bacteria, a pathogenic (disease-causing, S-Strain) and a nonpathogenic one (R-Strain). S-Strain: smooth presence due to the presence of capsule outside the cell wallR-Strain: lacked the capsule *capsule made the cell pathogenic*Mouse Injected with S-Strain (Pathogenic Control): Mouse DiesMose Injected with R-Strain (Nonpathogenic Control): Mouse is HealthyMouse Injected with Heat-Killed S Cells (Nonpathogenic Control): Mouse is HealthyMixture of Heat-Killed S Cells and Living R Cells: Mouse DiesGriffith found that the R-Strain has changed to S-KillingSomething from S-Strain entered living R-Strain and altered the genetic makeup (no one knew that DNA was what transferred)
Watson and Crick
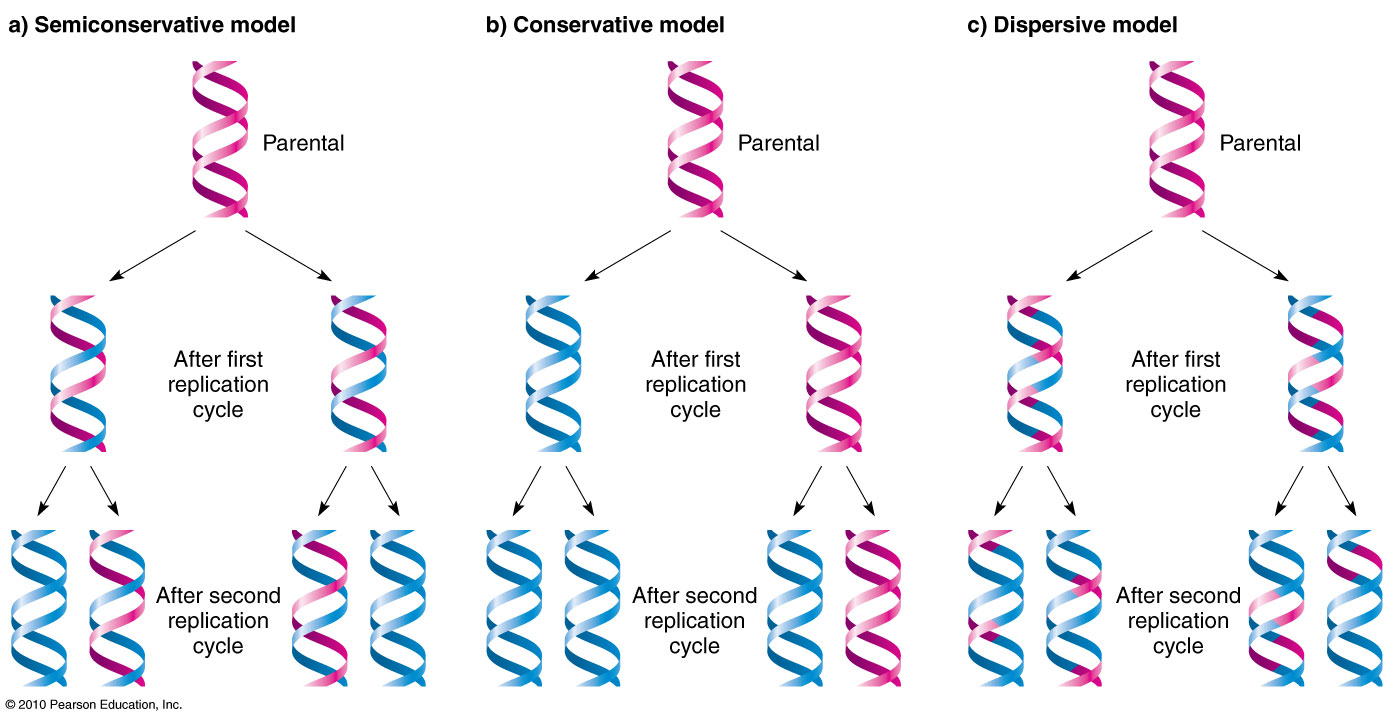
They came up with the double helix model of DNA; this structure satisfies the x-ray image if the two strands were antiparallel. They used the semi-conservative model to represent DNA, but there were other DNA models that competed.A) Conservative Model: Two parent strands reassociate after acting as templates for new strands, thus restoring the parental double helixB) Semi-conservative: Two stands of the parental molecule separate, and each function as a template for the synthesis of a new complementary strandC) Dispersive: Each strand of both daughter molecules contains a mixture of old and newly synthesized DNA.
Chargaff's Rule
Amount of Adenine = Amount of ThymineAmount of Guanine = Amount of Cytosine
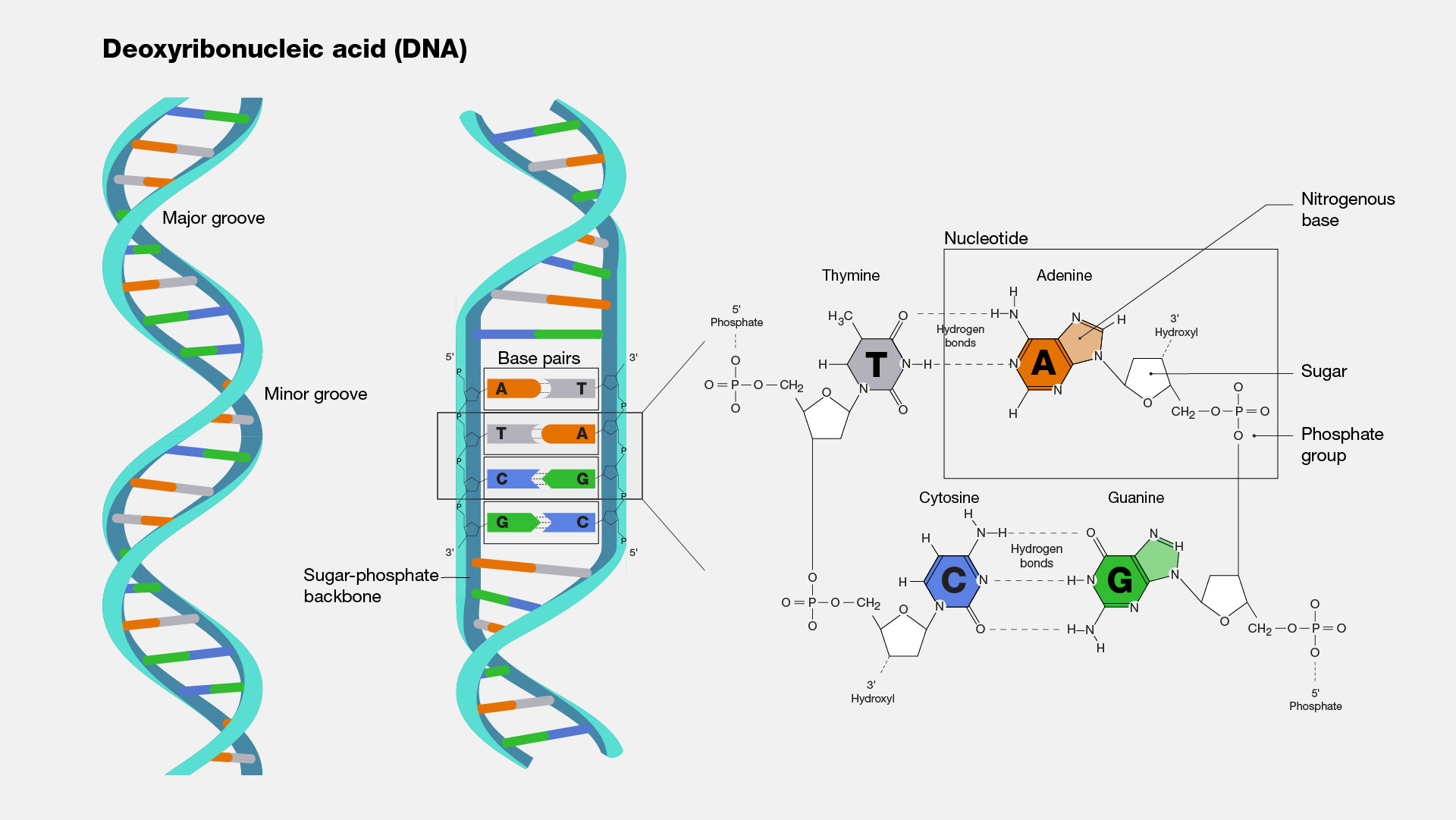
Structure of DNA Strand
Possible Base Pairings in
DNA Double Helix
Purine + Purine: Too widePyrimidine + Pyrimidine: Too narrowPurine + Pyrimidine: width is consistent with X-ray dataNitrogenous Base Pairs are held together by hydrogen bonds
Messleson and Stahl
Used to find model of DNA replication: Bacterial cultures are grown in regular 14N and 15N (controls).After growing 15N in several rounds of bacteria, they were transferred to medium containing 14N. They were grown for 20 mins -> bacteria is removed -> DNA is extracted from these bacteria and subjected to centrifugation with CsCl -> DNA is combined with CsCl and spun at high speeds -> resulted in a gradient of CsCl concentrations.The density of CsCl was highest at the bottom of the tube and the lowest at the top. DNA in this solution formed a band at the point in the tube where the density of CsCl corresponded with its density. Three bands of distribution are possibleHigh density (top of tube)Intermediate density (middle of tube)Low Density (top of tube)

Messleson and Stahl Conclusion
Hershey and Chase
In 1953, Hershey and Chase used bacteriophages (these transfer their genetic material into bacteria cells and reprogram the cells to make more bacteriophages, made of two components: proteins and DNA) to determine what was injected into bacterial cells. Using two tubes, they labeled one tube with 32P (radioactive phosphorus) and 35S (radioactive sulfur). They infected bacteria with 35S and another set of bacteria with bacteriophages with 32P. These two were mixed into a tube and later shaken to release bacteriophages from the bacterial surface. They then centrifuged the bacterial cells and looked for radioactivity in the supernatant and pellet. They found that radioactivity was only present inside the bacteria cells when they only used 32P (DNA). This indicated that DNA was injected inside bacteria and not protein.
Replication
We first start off with double stranded DNA (dsDNA) and create a replication bubble. This replication bubble happens at the origin of replication (sequence of nucleotides, aka ORI). The replication bubble creates two forks at each end of the bubble, and now each separated stand has to be used as a parent strand to form complementary daughter stands at thee ORI, suggested by the semiconservative model. DNA is separated at the ORI with the help of helicase, which separates the two strands to form the replication bubble. SSB's (single stranded proteins) keep the DNA single stranded. Topoisomerase helps relieve any strain caused by the unwinding of DNA. DNA Polymerase III: enzyme that adds nucleotides only to the 3' end, adds complementary base to daughter strand, needs RNA primer to add nucleotides, needs help of another protein called sliding clamp, contains two DNA polymerases in bacterial replication (DNA poly III and DNA poly I)Synthesis of Leading Strand: Uses Primase and DNA Polymerase IIIDNA Polymerase synthesizes leading strand towards replication fork and only one primer is required to synthesize the leading strand, created in the 5' to 3' directionLagging Strand Synthesis: Multiple RNA primers have to be laid down and then extended by DNA poly III to form short Okazaki fragments. These RNA Primers are removed by DNA poly I and are replaced with DNA nucleotides. Enzyme Ligase seals any gaps by connecting nucleotides by phosphodiester linkages

Transcription and Gene Regulation
Transcription in Eukaryotes
Transcription in Eukaryotes requires RNA Polymerase to make RNA nucleotides for pre-mRNA. The RNA Polymerase II transcribes the DNA from 3' to 5' to make the antiparallel pre-mRNA strand from 5' to 3'.Transcription in eukaryotes occurs in the nucleus of the cell.RNA ProcessingThe reason transcription in eukaryotes makes pre-mRNA instead of regular mRNA like prokaryotes, they have introns that do not express any genes and they need to be removed. Additionally, the newly formed pre-mRNA needs to exit the nucleus to be translated by free ribosomes and it needs to be protected from degradation in the environment so... Then spliceosomes come in to remove the introns and connect the exons, at this point, the pre-mRNA is then matured in to mature mRNA and is ready to exit the nucleus.
Initiation
A Eukaryote has a promoter usually consisting of As and Ts called the TATA box upstream of the start point of transcription.Transcription factors bind to the DNA strand so RNA polymerase binds to the promoter.RNA Polymerase unwinds the DNA and transcribes the DNA from 3' to 5' to make the pre-mRNA 5' to 3'.
Elongation
This is where RNA polymerase II adds the complementary RNA nucleotide to the correct nucleotide base in the 5' to 3' direction.This processes downstream from the start point of transcription.
Termination and
RNA Processing
TerminationThis process occur within the nucleus still and adds the 5' G cap on the 5' end of the pre-mRNA and a poly A tail to the 3' end.The poly A tail needs a sequence , AAUAAA, to add the tail with the help of poly A polymerase.RNA ProcessingThe reason transcription in eukaryotes makes pre-mRNA instead of regular mRNA like prokaryotes, they have introns that do not express any genes and they need to be removed. Additionally, the newly formed pre-mRNA needs to exit the nucleus to be translated by free ribosomes and it needs to be protected from degradation in the environment.Then spliceosomes come in to remove the introns and connect the exons, at this point, the pre-mRNA is then matured in to mature mRNA and is ready to exit the nucleus.
Gene Regulation in Eukaryotes
Regulation can occur at any point of the process from making DNA into pre-mRNA in transcription or by making proteins in translation.There are two types of expression of a gene in regulation:Basal: Low-level expression which results in low level DNA transcription, this results in the mRNA made leaving the nucleus and instantaneously being degraded.High Level: High level expression which allow the mRNA made to be used to make a protein through translation.To allow for these types of expression, there are transcription factors that have different types of expression...General leads to basal transcription of DNA Specific leads to changing the level of transcription from low to high.Needs activators to bind the distal control elements, enhancers, to increase the transcription level.A DNA bending protein will then bend the DNA strand to make the activator closer to the promoter where mediators and specific transcription factors are bound to it.The activators are then bound to the mediatorRNA polymerase II is then recruited to transcribe the DNA.
Transcription in Prokaryotes
The process of transcription in prokaryotes is the same as eukaryotes except some unique features The location of transcription for prokaryotes is in the cytoplasm of the cell since there is no nucleus in prokaryotic cells.They use RNAP which is RNA polymerase.No 5' cap nor poly A tail is necessary.Additionally, there is no RNA processing in prokaryotes since translation occurs simultaneously as DNA is transcribed.
Gene Regulation in Prokaryotes
A prime example of gene regulation in prokaryotes is with the lac operon.This regulation occurs in transcription.There are two types of regulationPositive which uses activator proteins to bind to the operator which allows for transcription to happen.And Negative which uses repressor proteins to bind to the operator which does not allow for transcription to occur.The operator is the on/off switch for expression and control operons which are clusters of genes.In the lac operon...lac, meaning lactose, will suggest that lactose is needed for this operon to work.It uses both positive and negative responses meaning both activators and repressor are used.Regulatory gene I is always active which codes for the repressor protein and is upstream from the promoter.The constitutive genes are lac Z which codes for beta galactosidase which breaks down lactose into glucose and galactose, lac Y which codes for permease to allow lactose into the cell, and beta galactoside-transacetylase.Repressors are always active so if glucose is not present, the repressor will bind to the promoter and expression be at the basal level.If lactose is present and glucose is not as present, the repressor will bind to allolactose and cAMP will bind to CAP and then bind to the promoter and the operon will be on, meaning there will be high expression.If glucose and lactose are highly present, glucose will block the production of cAMP through blocking adenylyl cyclase and through the transitive property, cAMP will not bind to CAP and thus not bind the promoter. This will ultimately lead to basal expression.
Translation
Translation in Eukaryotic
This occurs in the cytoplasm of the cell. Uses mRNA and tRNA to connect amino acids so protein can be made. This protein is later used in different parts of the cell or throughout the body.
Initiation
tRNA is made. TRNA- a single R N A strand, this anticodon binds the codon (5’ to 3’) bring the correct amino acid to be added during translation. Amino acid and the appropriate tRNA enter the active site of the specific synthetase, Tyrosyl-tRNA synthetase. Uses ATP to create covalent bonding between both. Finally is released to work in eleongationmRNA is decoded to produce the specific sequence of amino acids in a polypeptide chain.TRNA binds with the small subunit, which is connected to the mRNA. It binds to the start codon AUG. Then, the large ribosomal subunit come along to form a translation initiation complex.
Elongation
Large subunit is composed of 3 sites E, P, and A. The P site holds the tRNA that carries the growing polypeptide chain. The A site holds the tRNA that carries the next amino acid to be added to the chain. The E site is the exit site, where discharged tRNA’s leave the ribosome. Amino acids are added from N to C direction. This is repetitive until there is a stop codon. The amino acids are connected by peptide bonds.
Termination
When amino acid reaches a stop codon (UAA, UAG, or UGA) translation is stopped. This causes the protein to be released. This allows the protein to take its own path based on what kind of protein was made.
Regular protein
The protein is released and it goes and works for either nucleus, peroxisomes, or the mitochondria.
Protein with ER signal
Contain a signal peptide that allows the ribosome to go towards the endoplasmic reticulum. The signal peptide is connected to the signal recognition particle. This brings the protein closer to the ER membrane. When it reaches the membrane, the ribosome is released, and the protein goes into the ER membrane. After this, it travels to the Golgi apparatus where it receives the information to go to the plasma membrane and complete its task.
Translation in Prokaryotes
Translation in prokaryotes is relatively the same as in eukaryotes. However, the start amino acid for them is f-met.The small subunit is connected to a section called Shine-Dalgarno.
Protein Modification
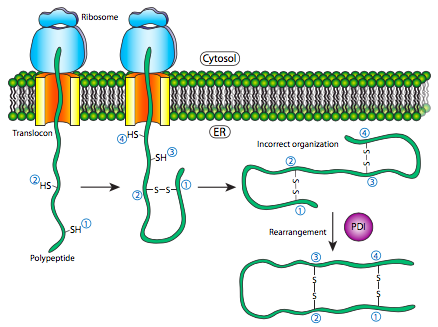
Folding
This is where the protein enters the ER and where a linear polypeptide folds into its characteristic and functional three dimensional structure. Amino acids in the ER react with each other to produce the three dimensional shape needed. This is now known as the native state. Failure to fold produces inactive proteins.
Glycosylation
Glycosylation is the process by which a carbohydrate is covalently attached to a target macromolecule, typically proteins and lipids. This modification serves various functions. Some proteins do not fold correctly unless they are glycosylated.
Multimeric Protein Assembly
Proteolytic Cleavage
Proteolytic cleavage is the process of breaking the peptide bonds between amino acids in proteins. This process is carried out by enzymes called peptidases, proteases or proteolytic cleavage enzymes.
Subtopic
Subtopic
Topic 2: Energy and Cell Communication
Signal Transduction
Starts with: Reception, then Transduction, and ends with a certain response.
Reception
Reception can rely on different receptor-ligand systems.
G-Protein Coupled Receptor (GPCR)
G-Protein Coupled Receptor (transmembrane protein):Types of Signal Molecules that use GPCR: Protein hormones, neurotransmitters, etc. GPCRs are all similar in structure; they typically bind to G ProteinsG-Proteins are named this because they interacted with Guanosime Diphosphate (GDP) or Guanosine Triphosphate (GTP). How GPCR Functions:Things Involved: GPCR, G-Protein, and an enzyme -- all inactive in the membraneG-Protein has the ability to bind both GDP and GTP, but when bound to GDP, it is in an inactive form. G-Protein is important because it needs to alter its shape to bind to GTP --> once GTP is bound, it can activate the enzymeHow Does the G-Protein Alter Its Shape to Bind to GTP: The signal molecule binds to GPCR.Once bound, there is a slight alteration in GPCR shape, this allows for G-Protein to bind to signal moleculeThis causes GDP (inactive when bound to G-Protein) to be replaced with GTP on G-ProteinG-Protein is now bound to GTP, which makes it activeActive G-Protein can now activate a nearby enzyme*This all happens at Reception*
Tyrosine Kinase Receptor
Tyrosine Kinase Receptor: Made of two polypeptides, these polypeptides dimerize when a signal molecule is bound to each polypeptideEach polypeptide has the ability to function as a kinase (an enzyme that adds phosphate groups to something). Each polypeptide on dimerization functions as a kinase --> takes phosphate groups from ATP and adds it to other polypeptides (autophorylation)Activated receptor can now interact with other proteins to bring response from cell*Phosphate groups are added to tyrosines --> referred to as Tyrosine Kinase Receptor)*
Ion Channel Receptor
Ligand-gated ion channels require a specific ligand to bind to the receptor in order to open the channel protein to allow certain ions like Ca+ and Na+ though.
Intracellular and Membrane
Receptors
Receptors can be on the cell membrane or within the cell, intracellular.Membrane receptors bind to large and/or polar ligands since they cannot passively diffuse across the membrane.Usually transmembrane proteinsCan be hormones, lipoproteins, extracellular matrix, neurotransmitters, and many other molecules bonded to itIntracellular receptors bind with small nonpolar ligands since they can diffuse past the membrane easily.A common example of this kind of reception deals with the steroid aldosterone.The hormone will bind to the intracellular receptor after diffusing across the membraneThe hormone-receptor system enters the nucleus through the nuclear pore to bind to a certain gene to act as a transcription factor.DNA is transcribed to mRNA then translated to a protein

Membrane Receptor

Intracellular Receptor
Transduction
Relay Molecules are converted to bring about a certain response through transduction.
Phosphorylation Cascade
Relay Molecule activates Protein Kinase (PK1)Then PK1 activated PK2 through the transfer of a phosphate from ATP, phosphorylation.This begins the cascade.PK2 activates an inactive protein through phosphorylation. In order to remove the phosphate off the protein kinase and active protein, a phosphatase is required and that is what PK2 and an active protein go through to convert them to their inactive forms.This process is necessary since the system does not need an excess amount of the response needed.
2ndary Messenger
cAMP
Cyclic AMP is a secondary messenger in transduction of the G Protein signaling pathway. Secondary messengers, mainly act to amplify the signal to render a greater response.When GTP/G Protein system binds to Adenylyl Cyclase, cAMP is made through phosphorylation of the enzyme with ATP coming from chemiosmosis in aerobic cell respiration.cAMP will then activate a Protein KinaseDepending on the process, there may be multiple protein kinases, the process may go through a phosphorylation cascade.
Response
Based on the transduction pathway, different responses arise.Let's take a look at the different responses to the epinephrine hormone...
Blood Sugar Increase
Epinephrine binds to a beta receptor on a liver cell.This results from glycogen breaking down in the reserves within the cell. Then to be released to effectually increase the blood sugar levels in the blood.
Blood Vessel
Dilation
Epinephrine binds to a beta receptor on a smooth muscle cell in the wall of a skeletal muscle blood vessel.The cell relaxes in order to allow more blood to flow with a dilated vessel to the skeletal muscle.
Blood Vessel
Constriction
Epinephrine binds to an alpha receptor on a smooth muscle cell in the wall of a intestinal blood vessel.The cell contracts in order to allow less blood to flow with a constricted vessel to the intestines.
Aerobic Cell Respiration
Glycolysis
Key ideas are:Step 1: Hexokinase puts a phosphate on a glucose from ATP to make it Glucose 6-phosphate, making it more reactive. Step 3: Phosphofructokinase phosphorylates Fructose 6-phosphate to make it into Fructose 1, 6-bisphosphate.Glucose is oxidized to make pyruvate as the end product as well as NADH and a small amount of ATP.
Pyruvate Oxidation
Pyruvate is oxidized to make Acetyl CoA.
Citric Acid Cycle
Key Ideas are: Step 1: Acetyl CoA binds with oxaloacetate to make citrateStep 3: Isocitrate is converted to alpha-Ketoglutarate and CO2 and NADHOverall, a miniscule amount of ATP is made as well as FADH2 and NADH is made to be electron carriers for the ETC.
Oxidative Phosphorylation
Oxidative Phosphorylation is broken up into two processes: The Electron Transport Chain (ETC) and ChemiosmosisIn the ETC:Electrons carried by NADH are sent to Complex I and e- carried by FADH2 is sent to Complex IIe- from complex I and II are sent to Q then Complex III.Then to Complex IV then to be finally accepted by O2 to make water.Complexes I, III, and IV are H+ pumps and the energy released by sending e- down the ETC powers the proton pump to intuitively pump H+ out into the intermembrane space.As a result of this chemiosmosis is able to happen.Since there are a lot of protons in the intermembrane space, a proton gradient will form to go back inside the matrix and to do this, an enzyme called ATP Synthase will perform facilitated diffusions and the energy of the gradient will activate the enzyme and will add a phosphate ADP to make ATP.This is especially important since this is the point where the most ATP is made in cell respiration.
Anaerobic Fermentation
Fermentation occurs when there is no O2 present within the system. Glycolysis still occurs though.
Alcohol
Pyruvate is converted to acetaldehyde and through NAD+ regeneration, ethanol is formed.
Lactic Acid
Pyruvate is converted to lactate through NAD+ regeneration.
ATP Formation
Process in which energy is made for the use of the cells.
Cell Respiration
The processed breakdown of glucose in order to create ATP.
Glycolosis
Step 7 of Glycolysis: 1-3 biphosphate glycerate needs a phosphate to be removed in order to become 3-phopshate glycerate. Phosphate is removed and it attaches to 2 ADP to form 2 ATP.Step 10 of Glycolysis: Phosphoenol-pyruvate needs to have a phosphate removed to turn into pyruvate. So pyruvate can have a function later in pyruvate oxidation. The phosphate group leaves and it attaches to 2 ADP to form 2 ATP.
Citric Acid Cycle
Succinyl CoA is turned into succinate which allows a phosphate to attach to GDP to turn it into GTP. When GTP is going to cycle back, ADP gets close and removes phosphate from GTP. This allows the phosphate to connect to ADP to create ATP.
Chemiosmosis
Part of Oxidation PhosphorylationAt first, complexes pump out H+ against their concentration gradient into the intermembrane space. (Occurring in electron transport chain)After that occurs, the intermembrane space gets packed with H+ so they go back in. When this occurs, the H+ cross with the help of a ATP Synthase. The energy used to cross the protein is used to add a phosphate to ADP. When the phosphate attaches to ADP it creates ATP.
Photosynthesis
The process in which a plant uses sun energy to create their own energy within.
Citric Electron Flow
Electrons need to be transferred from the primary receptor to the p700 chlorophyll molecules to continue the process and create NADPH. Sometimes, there is too much NADPH present, so the electrons from the acceptor are transferred to FD. From there, they go to the cytochrome complex, the constant movement of the electron causes the formation of ATP.
Connections
Transcr/
Translat
Animal cell part with ER connect to translation using the ER.A Common component of the cell is a ribosome, and they are known for making proteins, so how do they do that, through translation.In prokaryotes, 5’cap and 3’ tail is not put on mRNA since translation happens simultaneously as DNA is transcribed into mRNA as opposed to the cap and tail being in eukaryotic mRNA to protect from the environmental dangers leaving the nucleus.
Glycoproteins
Protein, Lipids, and Carbs connect to Glycosylation. They are all used. Carbs are attached to proteins or lipids to create glycoproteins or glycolipids.
Cell Resp/
Photosynth
Chemiosmosis connects to passive transport/osmosis because the movement of h+ down their concentration gradient.Energy for active transport is required but it does not have to be ATP, it could be from electrons being sent down the electron transport chain through the different complexes.The mitochondria and the chloroplast produce ATP similarly in which they both pump H+ against the conc. gradient. In both of these processes, the energy associated with this is used to add inorganic phosphate to ADP to form ATP.Protein pumps are used in cellular respiration to transport protons into the inter-membrane space.Photosynthesis and cellular respiration are reversed reactions to each other so knowing one, you essentially know the other.
DNA Structure
In nucleic acid, they have phosphorus and in proteins, they can have sulfur; using that knowledge, we can connect the Hershey and chase experiment to using radioactive phosphorus and sulfur respectively to see what was concentrated within the bacterium.
Signal Transduction
Signal transduction pathways are required to create a response that could result In bulk transport.Adenylyl cyclase is activated by g-proteins, which helps catalyze cAMP.