Biology 311C Book
Chapter 1
Section 1.1
THEME: New properties emerge at each level in the biological hierarchy
emergent properties - new properties that arise with each step upward in the hierarchy of life, owing to the arrangement and interactions of parts as complexity increases
ex: although photosynthesis occurs in an intact chloroplast, it will not take place in a disorganized test-tube mixture of chlorophyll and other chloroplast molecules. Because photosynthesis requires a specific organization of these molecules in the chlroplast
ex: if a blow to the head disrupts the intricate architecture of a human brain, the mind may cease to function properly even though all the brain tissues are still present. Our thoughts and memories are emergent properties of a complex network of nerve cells
reductionism - the approach of reducing complex systems to simpler components that are more manageable to study
ex: by studying the molecular structure of DNA that had been extracted from cells, James Watson and Francis Crick inferred, in 1953, how this molecule could serve as the chemical basis of inheritance
systems biology - approach that attempts to model the dynamic behavior of whole biological systems based on a study of the interactions among the system's parts.
a combination of components that function together
ex: ground level on a street corner, where you can observe local traffic, to a helicopter high above a city, from which you can see how variables such as time of day, accidents, etc. affect traffic throughout the city
[1-10. 1 = Largest, 10 = Smallest]
1) Biosphere - all life on earth and all the places where life exists
2) Ecosystems - all living things in a particular area, along with all the nonliving components of the environment with which life interacts
3) Communities - entire array of organisms inhabiting a particular ecosystem
4) Populations - all the indibiduals of a species living within the bounds of a specified area
5) Organisms - individual living things
6) Organs and Organ Systems - organs are body parts that carries out a particular funtion in the body. Organ systems are a team of organs that cooperate in a larger function
7) Tissues - a group of cells that work together, performing a specialized function
8) Cells -life's fundamental unit of structure and function. Single cell performs all functions of life. Multi-cellular divides labor among spcialized cells
9) Organelles - the various functional components present in cells
10) Molecules - chemical structure consisting of two or more small chemical units called atoms
THEME: Organisms Interact with Other Organisms and Physical Environment
ex: leaves absorb light energy from the sun
ex: leaves fall to the ground and are decomposed by organisms that return minerals to the soil
ex: water and minerals in the soil are taken up by the tree through its roots
ex: animals eat leaves and fruit from the tree
ex: leaves take in carbon dioxide from the air and release oxygen
global climate change - increase in temperature and change in weather patterns all around the planet, due mostly to increasing atmospheric CO2 levels from the burning fossil fuels. The increase in temperature, called global warming, is a major aspect of global climate change
THEME: Life requires energy transfer and transformation
Similar to the examples under theme to the left. All are related and dependent on one another, forming an ecosystem
a fundamental characteristic of living organisms is their use of energy to carry out ife's activities
THEME: Structure and Function Are Correlated at All Levels of Biological Organization
ex: a leaf; its thin, flat shape maximizes the amount of sunlight that can be captured by its chloroplasts. Analyzing a iological structure gives us clues about what it does and how it works. Conversely, knowing the function of something provides insight into its construction
THEME: The Cell is An Organism's Basic Unit of Structure and Function
Cell has a special place as the lowest level of organization that can perform all activities required for life
eukaryotic cell - a cell subdivided by internal membranes into various membrane-enclosed organelles
largest organelle is nucleus
The other organelles are located in the cytoplasm, the entire region between the nucleus and outer membrane of the cell
prokaryotic cell - a cell where the DNA is not separated from the rest of the cell by enclosure in a membrane-bounded nucleus
lack the other kinds of membrane-enclosed organelles that characterize eukaryotic cells
THEME: The Continuity of Life is Based on Heritable Information in hte Form of DNA
genes - the units of inheritance that transmit information from parents to offspring
DNA - deoxyribonucleis acid; chromosomes that have almost all of the cell's genetic material
the molecular structure of DNA accounts for its ability to store information. Each DNA molecule is made up of two long chains, called strands, arranged in a double helix
DNA provides the blueprints for making proteins, and proteins are the main players in building and maintaining the cell and carrying out its activities
Other human proteins include proteins in a muscle cell that drive contraction and the defensive proteins called antibodies. Enzymes, which catalyze (speed up) specific chemical reactions, are mostly proteins and are crucial to all cells
gene expression - the procee in which a sequence of nucleotides along a gene is transcribed into RNA, which is then translated into a specific protein with a unique shape and function. The information in a gene directs the production of a cellular product
a particular sequence of nucleotides says the same thing in one organism as it does in another
differences between organisms reflect differences between their nucleotide sequences rather than between their genetic codes
genome - the entire "library" of genetic instructions that an organism inherits
genomics - the science of studying whole sets of genes of a species as well as comparing genomes between species
bioinformatics - the use of computational tools to store, organize, and analyze the huge volume of data that result from high-throughput methods
THEME: Feedback Mechanisms Regulate Biological Systems
Just as a coodrinated control of traffic flow is necessary for a city to function smoothly, regulation of biological processes is crucial to the operation of living systems. The key is the ability of many biological processes to self-regulate by a mechanism called feedback
negative feedback - accumulation of an end product of a process slows that process
most common form of regulation in living systems
ex: the cell's breakdown of sugar generates chemical energy in the form of a substance called ATP. When a cell makes more ATP than it can use, the excess ATP "feeds back" and inhibits an enzyme near the beginning of the pathway
positive feedback - an end product speeds up its own production
ex: the clotting of your blood in response to injury. When a blood vessel is damages, structures in the blood called platelets begin to aggregate at the site. Positive feedback occurs as chemicals released by the platelets attract more platelets. The platelet pileup then initiates a complex process that seals the qound with a clot
Section 1.2
CORE THEME: Evolution accounts for the unity and diversity of life
THEME: Classifying the Diversity of Life
Three domains of life:
Bacteria
all prokaryotic
Chapter 27 explains differences
bacteria are the most diverse and widespread prokaryotices and are now classified into multiple kingdoms
Archaea
all prokaryotic
Many of the prokaryotes knowns as archaea live in the Earth's extreme environments, such as salty lakes and boiling hot springs. Domain Archaea includes multiple kingdoms
Eukarya
Includes three kingdoms of multicellular eukaryotes:
Plantae
produce their own sugars and other food molecules by photosynthesis
Fungi
absorb dissolved nutrients from their surroundings; many decompose dead organisms and organic wastes and obsorb nutrients from these sources
Animalia
animals obtain food by ingestion, which is hte eating and digesting of other organisms
THEME: Charles Darwin and the Theory of Natural Selection
"The Origin of Species" articulated two main points:
1st) that contemporary species arose from a succession of ancestors, an idea that Darwin supported with large amount of evidence
Darwin called this evolutionary history of species "descent of modification"
2nd) a proposed mechanism for descent with modification, called "natural selection"
natural selection - when the natural environment "selects" for hte propagation of certain traits among naturally occurring variant traits in the population
Darwin propsed that natural selection, by its cumulative effects over long periods of time, could cause an ancestral species to give rise to two or more descendant species. This could occur, for example, if one population fragmented into several subpopulations isolated in different environments. In these separate arenas of natural selection, one species could gradually radiate into multiple species as the geographically isolated populations adapted over many generations to different sets of environmental factos
Section 1.3
CORE THEME: In studying nature, scientists make observations and then form and test hypotheses
science - a way of knowing, an approach to understanding the natural world
inquiry - the heart of science; a search for information and explanation, often focusing on specific questions
THEME: Making Observations
observation - use of the senses to gather information, either directly or indirectly with the help of tools such as microscopes that extend our senses
data - recorded observations
qualitative data - data in the form of recorded descriptions rather than numerical measurements
quantitative data - data generally recorded as measurements
inductive reasoning - logic of collecting and analyzing observations hopefully leading to important conclusions
Through induction, we derive generalizations from a large number of specific observations. "The sun always rises in the east" is an example. And so is "All organisms are made of cells"
THEME: Forming and Testing Hypotheses
hypothesis - a tentative answer to a well-framed question; an explanation on trial. It is usually a rational accounting for a set of observations, based on the available data and guided by inductive reasing
example of scientific thought process:
Observations
Question
Hypothesis 1: Dead batteries
Prediction: Replacing batteries will fix problem
Test of prediction
Test falsifies hypothesis
Hypothesis 2: Burnt-out bulb
Prediction: Replacing bulb will fix problem
Test of prediction
Test does not fasify hypothesis
deductive reasoning - generally used after the hypothesis has been developed and involves logic that flows in the opposite direction, from the general to the specific
ex: From general premises we extrapolate to the specific results we should expect if hte premises are true:
Premise 1: all organisms are made up of cells
Premise 2: humans are organisms
Conclusion from deductive reasoning = humans are composed of cells
THEME: The Flexibility of the Scientific Method
controlled experiment - an experiment that is designed to compare an experimental group with a control group
researchers usually "control" unwanted variables not by eliminating them through environmental regulations, but by canceling out their effects by using control groups
theory - an explanation that is broader in scope than a hypothesis, generates new hypothesis, and is supported by a large body of evidence
a theory is general enough to spin off many new, specific hypotheses that can be tested
compared to any one hypothesis, a theory is generally supported by a much greader body of evidence
Section 1.4
CORE THEME: Science benefits from a cooperative approach and diverse viewpoints
THEME: Building on the work of others
model organism - a species that is easy to grow in the lab and lends itself particularly well to the questions being investigated
because all organisms are evolutionarily related, lessons learned from a model organism are often widely applicable
THEME: Science, Technology, and Society
technology - applies scientific knowledge for some specific purpose
science - the goal of science is to understand natural phenomena
Chapter 2
Section 2.1
THEME: Matter consists of chemical elements in pure form and in combinations called compounds
matter - anything that takes us space and has mass
element - substance that cannot be broken down to other substances by chemical reactions. Matter is made up of elements
compound - a substance consisting of two or more different elements combined in a fixed ratio
essential elements - elements that an organism needs to live a healthy life and reproduce
trace elements - elements required by an organism in only minute quantities
Section 2.2
THEME: An element's properties depend on the structure of its atoms
atom - the smallest unit of matter that still retains the properties of an element
Subatomic particles
neutrons
protons
electrons
electron shells - electrons found here. Each shell has a characteristic average distance and energy level
first shell closest to nucleus and has lowest potential energy
the chemical behavior of an atom is determined by the distribution of electrons in the atom's electron shells
valence electrons - the electrons on the outermost shell. The chemical behavior of an atom depends mostly on these
valence shell - the outmost electron shell
orbital - the three-dimensional space where an electron is found 90% of the time
dalton - the unit of measurement for atoms and subatomic particles
Isotopes - when an atom takes on a different atomic form that has more neutrons than other atoms of the same element
radioactive isotope - an isotope in which the nucleus decays spontaneously, giving off particles and energy
energy - defined as the cpaacity to cause change
potential energy - energy that matter possesses because of its location or structure
Section 2.3
THEME: The formation and function of molecules depnd on chemical bonding between atoms
chemical bonds - when atoms stay close together, held by attractions. Atoms with incomplete valence shells can interact with certain other atoms in such a way that each partner completes its valence shell: the atoms either share or transfer valence electrons
covalent bond - the sharing of a pair of valence electron by two atoms

two or more atoms held together by covalent bonds constitute a molecule
single bond - H-H or H:H, a pair of shared electrons
double bond - sharing two pairs of valence electrons O=O
valence - the number of unpaired electrons required to complete the atom's outermost shell
electronegativity - the attraction of a particular atom for the electrons of a covalent bond. The more electronegative an atom is, the more strongly it pulls shared electrons toward itself
nonpolar covalent bond - a type of covalent bond in which electrons are shared equally between two atoms of similar electronegativity

polar covalent bond - a covalent bond between atoms that differ in electronegativity. The shared electrons are pulled closer to the more electronegative atom, making it slightly negative and the other atom slightly positive

Subtopic
ionic bond - the attraction of cations and anions forming a bond

ion - a charged atom or molecule
cation - when the charge is postitive
anion - when the charge is negative
ionic compounds/salts - compounds formed by ionic bonds
Hydrogen bonds - the noncovalent attraction between a hydrogen and an elctronegative atom

the partial positive charge on a hydrogen atom allows the hydrogen to be attracted to a different electronegative atom nearby
van der Waals interactions - weak attractions between molecules or parts of molecules that result from transient local partial charges
many small positive and negative attractions that, when put together, are strong
ex: each gecko toe has hundreds of thousands of tiny hairs, with multiple projections at each hair's tip that increase surface area. The van der Waals interactions between the hair tip molecules and the molecules of the wall's surface are so numerous htat despite their individual weakenss, together they can support the gecko's body weight
Section 2.4
THEME: Chemical reactions make and break chemical bonds
chemical reactions - the making and breaking of chemical bonds, leading to changes ni the composition of matter
Stoichiometry
reactants - starting materials
products - ending materials
chemical equilibrium - the point at which the reactions exactly offset one another
Chapter 3
Section 3.1
THEME: Polar covalent bonds in water molecules result in hydrogen bonding
polar covalent bond - a covalent bond between atoms that differ in electronegativity. The shared electrons are pulled closer to the more electronegative atom, making it slightly negative and the other atom slightly positive
ex: [Polar covalent bonds in a water molecule] because oxygen is more electronegative than hydrogen, shared electrons are pulled more toward oxygen. This results in a partial negative charge on the oxygen and a partial positive charge on hte hydrogens
polar molecule - a molecule (such as water) with an uneven distribution of charges in different regions of the molecule
Section 3.2
THEME: Four emergent properties of water contribute to Earth's suitability for life
Cohesion of water molecules
cohesion - the linking together of like molecules by hydrogen bonds
surface tension - a measure of how difficult it is to stretch or break the surface of a liquid
water has a greater surface tension than most other liquids. At the interface between water and air is an ordered arrangement of water molecules, hydrogen-bonded to one another and to the water below
adhesion - the clinging of one substance to another
Moderation of temperature by water
kinetic energy - the energy of motion
for a given body of matter, the amount of heat is a measure of the matter's total kinetic energy due to the motion of its molecules; thus, heat depends in part on the matter's volume
temperature - measure of heat intensity that represents the average kinetic energy of the molecules, regardless of volume
calorie - the amount of heat it takes to raise the temperature by 1 g of water by 1 * C (degrees celcius)
temperature is the average kinetic energy of molecules
specific heat - the amount of heat that must be absorbed or lost for 1 g of that substance to change its temperature by 1 * C (degrees celcius)
we can trace water's high specific heat, like many of its other properties, to hydrogen bonding. Heat must be absorbed in order to break hydrogen bonds; by the same token, heat is released when hydrogen bonds form
heat of vaporization - the quantity of heat a liquid must absorb for 1 g of it to be converted from the liquid to the gaseous state
water's high heat of vaporization is another emergent property resulting from the strength of its hydrogen bonds, which must be broken before the molecules make their exodus from the liquid
evaporative cooling - as a liquid evaporates, the surface of the liquid that remains behind cools down
Water: the solvent of life
solution - a liquid that is a completely homogeneous mixture of two or more substances
solvent - the dissolving agnet of a solution
solute - substance that is dissolved
An aqueous solution is one in which water is the solvent
hydration shell - the sphere of water molecules around each dissolved ion
ex: we have a spoonful of table salt, the ionic compound sodium chloride (NaCl). Placed in water. Surface of each grain, or crystal, of salt, the sodium and chloride ions are exposed to the solvent. These ions and the water molecules have a mutual affinity owing to the attraction between opposite charges. The oxygen regions of the water molecules are negatively charged and are attracted to sodium cations. The hydrogen regions are positively charged and are attracted to chloride anions. As a result, water molecules surround the individual sodium and chloride ions, seperating and shielding them from one another
Working inward from the surface of each salt crystal, water eventually dissolved all ions. The result is a solution of two solutes, sodium cations and chloride anions, homogeneously mixed with water, the solvent
hydrophillic - an substance that has an affinity to water
substances can be hydrophilic without actually dissolving
ex: some molecules in cells are so large that they do not dissolve. Instead they remain suspended in the aqueous liquid of the cell
colloid - a stable suspension of fine particles in a liquid
hydrophobic - a substance that can repel water
cannot form hydrogen bonds
ex: vegetable oil
Section 3.3
Acidic and basic conditions affect living organisms
hydrogen ion - H+, a single proton with a charge of 1+
Occasionally, a hydrogen atom participating in a hydrogen bond between two water molecules shifts from one molecule to the other. When this happens, the hydrogen atom leaves its electron behind, and what is actually transferred is a hydrogen ion
The water molecule that lost a proton is now a hydroxide ion (OH-), which has a charge of 1-
The proton binds to the other water molecule, making that molecule a hydronium ion (H3O+)
chem reaction: 2 H2O => H3O+ + OH-
double arrows means reversible reaction that reaches a state of dynamic equilibrium
Acids and Bases
acid - a substance tht increases the hydrogen ion concentration of a solution
ex: HCl -> H+ + Cl-
this source of H+ (dissociation of water is the other source) results in an acidic solution - one having more H+ than OH-
base - a substance that reduces the hydrogen ion concentration of a solution
ex: NH3 + H+ <=> NH4+
the base reduces the H+ concentration. Solutions with a higher concentration of OH- than H+ are known as basic solutions
a solution in which the H+ and OH- concentrations are equal is said to be neutral
pH declines as H+ concentration increases
a solution of pH 3 is not twice as acidic as a soution of pH 6, but a thousand times more acidic
Buffers
buffer - a substance that minimizes changes in hte conentrations of H+ and OH- in a solution
it does so by accepting hydrogen ions from the solution when they are in excess and donating hydrogen ions to the solution when they have been depleted
There are several buffers that contribute to pH stability in human blood and many other biological solutions. One of these is carbonic acid (H2CO3), formed when CO2 reacts with water in blood plasma. Carbonic acid dissociates to yield a bicarbonate ion (HCO3-) and a hydrogen ion (H+):
H2CO3 <=> HCO3- + H+
the chemical equilibrium between carbonic acid and bicarbonate acts as a pH regulator, the reaction shifting left or right as other processes in the solution add or remove hydrogen ions
If the H+ concentration in blood begins to fall (that is, if pH rises), hte reaction proceeds to the right and more carbonic acid dissociates, replenishing hydrogen ions
But when H+ concentration in blood begins to rise (when pH drops), the reaction proceeds to the left, with HCO3- (the base) removing the hydrogen ions from the solution and forming H2CO3
the carbonic acid-bicarbonate buffering system consists of an acid and a base in equilibrium with each other. Most other buffers are acid-base pairs
Chapter 4
Section 4.1
THEME: Organic chemistry is the study of carbon compounds
Organic compounds range from simple molecules, such as methane (CH4), to colossal ones, such as proteins, with thousands of atoms. Most organic compounds contain hydrogen atoms in addition to carbon atoms
Section 4.2
THEME: Carbon atoms can form diverse molecules by bonding to four other atoms
hydrocarbons - organic molecules consisting of only carbon and hydrogen
atoms of hydrogen are attached to the carbon skeleton wherever electrons are available for covalent bonding
isomers - compounds that have the same numbers of atoms of the same elements but different structures and hence different properties
structural isomers - isomers that differ in the covalent arrangments of their atoms

cis-trans isomers (geometric isomers) - carbons that have covalent bonds to the same atoms, but these atoms differ in their spatial arrangements due to the inflexibility of double bonds
Single bonds allow the atoms they join to rotate freely about hte bond axis without changing the compound
In contrast, double bonds do not permit such rotation
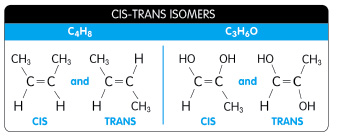
Notice CIS is on the left and TRANS is on the right
cis isomer: the two X's are on the same side
trans isomer: the two X's are on opposite sides
enantiomers - isomers that are mirror images of each other and that differ in shape due to the presence of an asymmetric carbon, one that is attached to four different atoms or groups of atoms

the "L" in "L-alanine" means left
the "D" in "D-alanine" means right
Section 4.3
THEME: A few chemical groups are key to the functioning of biological molecules
functional groups - chemical groups that affect molecular function by being directly involved in chemical reactions
7 chemical groups most important in biological processes:
hydroxyl - (---OH), a hydrogen atom is bonded to na oxygen atom, which is turn is bonded to a carbon skeleton of the organic molecule
compounds: alcohols (their specific names usually end in -ol)
functional properties: is polar as a result of the electrons spending more time near the electronegative oxygen atom. Can form hydrogen bonds with water molecules, helping dissolve organic compounds such as sugars
carbonyl group - (C=O) consists of a carbon atom joined to an oxygen atom by a double bond
compounds: ketones - if hte carbonyl group is within a carbon skeleton. Aldehydes - if the carbonyl group is at the end of the carbon skeleton
functional properties: a ketone and an aldehyde may be structural isomers with different properties, as is the case for acetone and propanal. Ketone and aldehyde groups are also found in sugars, giving rise to two major groups of sugars: ketoses (containing ketone groups) and aldoses (containing aldehyde groups)
carboxyl group - (---COOH) when an oxygen atom is double-bonded to a carbon atom that is also bonded to an ---OH group
compounds: carboxlic acids (organic acids)
functional properties: acts as an acid; can donate an H+ because the covalent bond between oxygen and hydrogen is so polar. Found in cells in hte ionized form with a charge of 1- and called a carboxylate ion
amino group - (---NH2) consists of a nitrogen atom bonded to two hydrogen atoms and to the carbon skeleton
compounds: amines. Glycine, a compound that is both an amine and a carboxylic acid because it has both an amino group and a carboxyl group; compounds with both groups are called amino acids
functional properties: acts as a base; can pick up an H+ from the surrounding solution (water, in living organisms). Found in cells in the ionized form with a charge of 1+
sulfhydryl group - (---SH) consists of a sulfur atom bonded to an atom of hydrogegn; it resembles a hydroxyl group in shape
compounds: thiols. Cysteine, an important sulfer-containing amino acid
functional properties: two sulfhydryl groups can react, forming a covalent bond. This "cross-linking" helps stabilize protein structure. Cross-linking of cysteines in hair proteins maintains the curliness or straightness of hair. Straight hair can be "permanently" curled by shaping it around curlers and then breaking and re-forming the cross-linking bonds
phosphate group - (---OPO3^2-) a phosphorus atom is bonded to four oxygen atoms; one oxygen is bonded to the carbon skeleton; two oxygens carry negative charges
compounds: organic phosphates. Glycerol phosphate, which takes part in many important chemical reaction in cells; glycerol phosphate also provides hte backbone for phospholipids, the most prevalent molecules in cell membranes
functional properties: contributes negative charge to the molecule which it is a part. Molecules containing phosphate groups have the potential to react with water, releasing energy
methyl group - (---CH3) consists of a carbon bonded to three hydrogen atoms. The carbon of a methyl group may be attached to a carbon or to a different atom
compounds: methylated compounds. 5-Mythyl cytosine, a component of DNA that has been modified by addition of a methyl group
functional properties: addition of a methyl group to DNA, or to molecules bound to DNA, affects the expression of genes. Arrangement of methyl groups in male and female sex hormones affects their shape and function
adenosine triphosphate - (ATP) consists of an organic molecule called adenosine attached to a string of three phosphate groups
Although ATP is sometimes said to store energy, it is more accurate to think of it as storing the potential to react with water
Chapter 5
OVERVIEW: The Molecules of Life
macromolecules - a giant molecule formed by the joining of small molecules, usually by a dehydration reaction. Polysaccharides, proteins, and nucleic acids are macromolecules
Section 5.1
THEME: Macromolecules are polymers, built from monomers
polymer - a long molecule consisting of many similar or identical building blocks linked by covalent bonds, much as a train consists of a chain of cars
monomers - the repeating units that serve as the building blocks of a polymer
monomers are connected by dehydration reactions
The Synthesis and Breakdown of Polymers
enzymes - specialized macromolecules that speed up chemical reactions
dehydration reaction - a reaction in which two molecules are covalently bonded to each other, with the loss of a water molecule
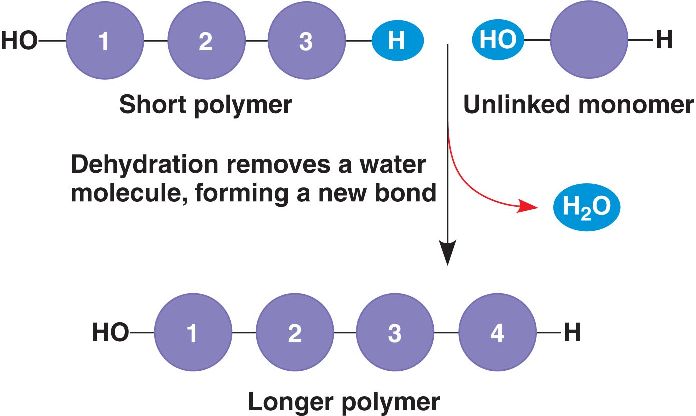
hydrolysis - a process that is essentially the reverse of the dehydration reaction. Polymers are disassembled to monomers by this

Section 5.2
THEME: Carbohydrates serve as fuel and building material
carbohydrates - both sugars and polymers of sugars
monosaccharide - the simplest carbohydrate, active alone or serving as a monomer for disaccharides and polysaccharides. Also known as simple sugars, monosaccharides have molecular formulas that are generally some multiple o CH2O
Subtopic
Chapter 25
Section 25.1
Conditions on early Earth made the origin of life possible
Scientists hypothesize that chemical and physical processes on early Earth, aided by the emerging force of natural selection, could have produced very simple cells through a sequence of four main stages:
1) The abiotic (nonliving) synthesis of small organic molecules, such as amino acids and nitrogenous bases
2) The joining of these small molecules into macromolecules, such as proteins and nucleic acids
3) The packaging of these molecules into protocells, droplets with membranes that maintained an internal chemistry different from that of their surroundings
4) The origin of self-replicating molecules that eventually made inheritance possible
Miller and Urey experiment - tested the Oparin-Haldane hypothesis by creating laboratory conditions comparable to those that scientists at the time thought existed on early earth
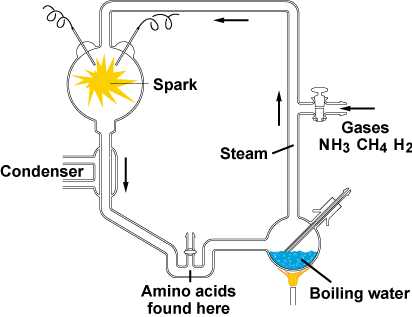
His apparatus yielded a variety of amino acids found in organisms today, along with other organic compounds. Many laboratories have since repeated Miller's classic experiment using different recipes for the atmosphere, some of which also produced organic compounds
Chapter 27
Section 27.1
Structural and functional adaptations contribute to prokaryotic success
most prokaryotes are unicellular, although the cells of some species remain attached to each other after cell division
most likely the first organism to inhabit earth (prokaryotes)
Subtopic
Cell-surface stuctures
a key feature of nearly all prokaryotic cells is the cell wall, which maintains cell shape, protects the cell, and prevents it from bursting in a hypotonic environment
in a hypertonic environment, most prokaryotes lose water and shrink away from their wall (plasmolyze), like other walled cells
such water losses can inhibit cell reproduction
Thus, salt can be used to preserve foods because it causes prokaryotes to lose water, preventing them from rapidly multiplying
the cell walls of prokaryotes differ in structure from those of eukaryotes. In eukaryotes that have cell walls, such as plants and fungi, the walls are usually made of cellulose or chitin
In contrast, most bacterial cell walls contain peptidoglycan, which is a polymer composed of modified sugars cross-linked by short polypeptides
Using a technique called the Gram stain, developed by the nineteenth-century Danish physician Hans Christian Gram, scientists can classify many bacterial species into two groups based on differences in cell wall composition
Samples are first stained with crystal violet dye and iodine, then rinsed in alcohol, and finally stained with a red dey such as safranin
The stucture of the bacterium's cell wall determines the straining response
Gram-positive bacteria have simpler walls with a relatively large amount of peptidoglycan
Gram negative bacteria have less peptidoglycan and are structurallly more complex, with an outer membrane that contains lipopolysaccharides (carbohydrates bonded to lipids)
The lipid portions of the lipopolysaccharides in the walls of many gram-negative bacteria are toxic, causing fever or shock
The outer membrane of a gram-negative bacterium helps protect it from the body's defenses
Gram-negative bacteria also tend to be more resistant than gram-positive species to antibiotics because the outer membrane impedes entry of hte drugs
The cell wall of many prokaryotes is surrounded by a sticky layer of polysaccharide or protein. This layer is called a capsule if it is dense and well-defined or a slime layer if it is less well organized
Both kinds of sticky outer layers enable prokaryotes to adhere to their substrate or to other individuals in a colony
Some prokaryotes stick to their substrate or to one another by means of hairlike appendages called fimbriae
Ex: the bacterium that causes gonorrhea uses fimbriae to fasten itslef to the mucous membranes of its host
fimbriae are usually shorter and more numerous than pili, appendages that pull two cells together prior to DNA transfer from one cell to the other; pili are sometimes referred to as sex pili
Motility
About half of all prokaryotes are capable of taxis, a directed movement toward or away from a stimulus
ex: prokaryotes that exhibit chemotaxis change their movement pattern in response to chemicals. They may move toward nutrients or oxygen (positive chemotaxis) or away from a toxic substance (negative chemotaxis)
Of the various structures that enable prokaryotes to move, the most common are flagella. Flagella may be scattered over the entire surface of the cell or concentrated at one or both ends
prokaryotic flagella differ greatly from eukaryotic flagella: they are one-tenth the wideth and are not covered by an extension of the plasma membrane
the flagella of prokaryotes are also very different in their molecular composition and their mechanism of propulsion
among prokaryotes, bacterial and archaeal flagella are similar in size and rotation mechanism, but the yare composed of different proteins
overall, these structural and molecular comparisons suggest that the flagella of bacteria, archaea, and eukaryotes arose independently
Since the flagella of organism in hte three domains perform similar functions but probably are not related by common descent, it is likely that they are analogous, not homologous, structures
Internal Organization and DNA
the cells of prokaryotes are simpler than those of eukaryotes in both their internal structure and the physical arrangement of their DNA
prokaryotic cells lack the complex compartmentalization found in eukaryotic cells. However, some prokaryotic cells do have specialized membranes that perform metabolic functions. These membranes are usually infoldings of the plasma membrane
the genome of a prokaryote is structurally different from a eukaryotic genome and in most cases has considerably less DNA
In the majority of prokaryotes, the genome consists of a circular chromosome with many fewer proteins than found in the linear chromosomes of eukaryotes
also, unlike eukaryotes, prokaryotes lack a membrane-bounded nucleus; their chromosome is located in the nucleoid, a region of cytoplasm that appears lighter than the surrounding cytoplasm in electron micrographs
in addition to its single chromosome, a typical prokaryotic cell may also have much smaller rings of independently replicating DNA molecules called plasmid, most carrying only a few genes
Reproduction and Adaptation
Prokaryotes are highly successful in part because of their potential to reproduce quickly in a favorable environment
by Binary Fission, a single prokaryotic cell divides into 2 cells, which then divide into 4, 8, 16, etc.
prokaryotic reproduction is limitied - the cells eventually exhaust their nutrient supply, poison themselves with metabolic wastes, face competition from other microorganism, or are consumed by other organisms
reproduction in prokaryotes draws attnetion to three key features of their biology
1) they are small
2) they reproduce by binary fission
as a result from these three features, prokaryotic populations can consist of many trillions of individuals - far more than populations of multicellular eukaryotes, such as plants and animals
3) they have short generation times
certain bacteria develop resistant cells called endospores when they lack an essential nutrient
the original cell produces a copy of its chromosome and surrounds it with a tough multilayered structure, forming the endospore
water is removed from the endospore, and its metabolism halts
the orginal cell then lyses, releasing the endospore
most endospores are so durable that they can survive in boiling water; killing them requires heating lab equipment to 121C under high pressure. In less hostile environments, endospores can remain dormant but viable for centuries, able to rehydrate and resume metabolism when their environment improves
Section 27.3
Diverse nutritional and metabolic adaptations have evolved in prokaryotes
the extensive genetic variation found in prokaryotic populations is reflected in the diverse nutritional adaptations of prokaryotes
Like all organisms, prokaryotes can be categorized by how they obtain energy and the carbon used in building the organic molecules that make up cells
Every type of nutrition observed in eukaryotes is represented among prokaryotes, along with some nutritional modes unique to porkaryotes
In fact, prokaryotes have an astounding range of metabolic adaptations, much broader than that found in eukaryotes
Organisms that obtain energy from light are called phototrophs
organism that obtain energy from chemicals are called chemotrpohs
organisms that need CO2 in some form as a carbon source are called autotrpohs
in contrast, heterotrophs require at least one organic nutrient, such as glucose, to make other organic compounds
the role of oxygen in metabolism
obligate erobes must use O2 for cellular respiration and cannot grow without it
obligate anaerobes, on the other hand, are poisoned by O2
some obligate anaerobes live exclusively by fermentation; others extract chemical energy by anaerobic respiration, in which substances other than O2, such as nitrate ions or sulfate ions, accept electrons at the "downhill" end of electron transport chains
facultative anaerobes use O2 if it is present but can also carry out fermentation or anaerobic respiration in an anaerobic environment
nitrogen metabolism
nitrogen is essential for the production of amino acids and nucleic acids in all organisms
whereas eukaryotes can obtain nitrogen from only a limited group of nitrogen compounds, prokaryotes can metabolize nitrogen in a wide variety of forms
for example, some cyanobacteria and some methanogens (a group of archaea) convert atmosphere nitrogen to ammonia, a process called nitrogen fixation
the cells can then incorporate this "fixed" nitrogen into amino acids and other organic molecules
in terms of their nutrition, nitrogen-fixing cyanobacteria are some of hte moset self-sufficient organisms, since they need only light, CO2, N2, water, and some minerals to grow
nitrogen fixation by prokaryotes has a large impact on other organisms
ex: nitrogen-fixing prokaryotes can increase the nitrogen available to plants, which cannot use atmospheric nitrogen but can use hte nitrogen compounds that the prokaryotes produce from ammonia
27.4

Chapter 8
8.1
the totality of an organism's chemical reactions is called metabolism
metabolic pathways - a series of chemical reactions that eitehr builds a comoplex molecule (anabolic pathway) or breaks down a complex molecule to simpler molecules (catabolic pathway)
catabolic and anabolic pathways are the "downhill" and "uphill" avenues of hte metabolic landscape
energy released from the downhilll reactions of catabolic pathways can be stored and then used to drive the uphill reactions of anabolic pathways
thermodynamics - the study of the energy transformation that occur in the collection of matter
first law - the energy of the universe is constant. "energy can be transferred and transformed, but it cannot be created or destroyed." The first law is also known as the "principle of conservation of energy."
second law - "every energy transfer or transformation increases the entropy of the universe."
a process that can occur without and input of energy is called a spontaneous process
"for a process to occur spontaneously, it must increase the entropy of the universe."
8.2
Gibbs free energy or just free energy - the portion of a system's energy that can perform work when temperature and pressure are uniform throughout the system, as in a living cell
change in G = change in H - T*change in S
change in H = enthalpy / change in S = entropy / T = absolute temperature in kelvin
once we know the value of change in G for a process, we can use it to predict whether the process will be spontaneous
processes with a negative change in G are spontaneous
change in G = G final state - G initial state
chemical reactions can be classified as:
exergonic (energy outward)
occur spontaneously
endergonic (energy inward)
Subtopic