Biomolecules
Proteins
Structural proteins: support bones, tendons, skin, claws, nails, and beaks
Transport proteins like the hemoglobin move molecules and ions across the cell membrane
Motor proteins like myosin and actin enable the movement of cells
Signalling proteins like insulin carry signals between cells
Receptor proteins like insulin receptors mediate a cell's response to a stimulus
Defense proteins like antibodies bind and inactivate foreign substances
Storage proteins like ovalbumin store amino acids for future use
Structural proteins like collagen and keratin provide protections and support
Enzymes act as catalysts
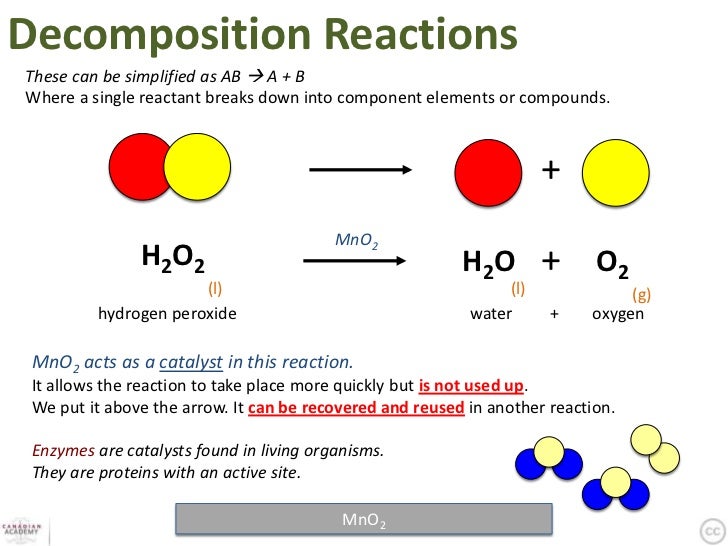
Protein catalysts speed up chemical reactions without being consumed in the process, but only if the reaction would have been able to take place even without the catalyst. They posses an activation energy (EA) that must be over come. Proteins are denatured at a high temperature.

a subtrate is the reactant that the enxyme acts on and binds at the active site (a groove in the 3D structure of the protein). The functional group comes into contact with the amino acid and forces the protein to reshape.
Enzyme Functions: Temperature and pH levels affect enzyme activity where increase in temperature will increase the speed of the catalyst until a critical point where the protein is denatured. Some enzymes require non-protein cofactors and organic co-enzymes before they can begin functioning. As an example NAD+ acts as an electron carrier in cellular respiration.
Enzyme inhibition: Competetive inhibitors are similar to an enzyme's substrate as they can enter the active site and stop the real substrate from binding.
Non-competetive inhibitors: attach to another site on the enzyme and change its shape
Allosteric Regulation: enzymes may possess allosteric receptor sites that simulate enzyme activity.
Binding an activator=Active enzyme
Binding an inhibitor= Stopping enzyme function
Monomers and Functional groups:Amino Acids (AA) (N,C,O and H). Central carbon bonded to an AA group (-Nh2), a carboxyl group (-COO), a H atom and an R group.

20 different AAs differ by unique chain R group (Remainder groups)
Since proteins are comprised of amino acids which are monomers, proteins themselves are polymers!
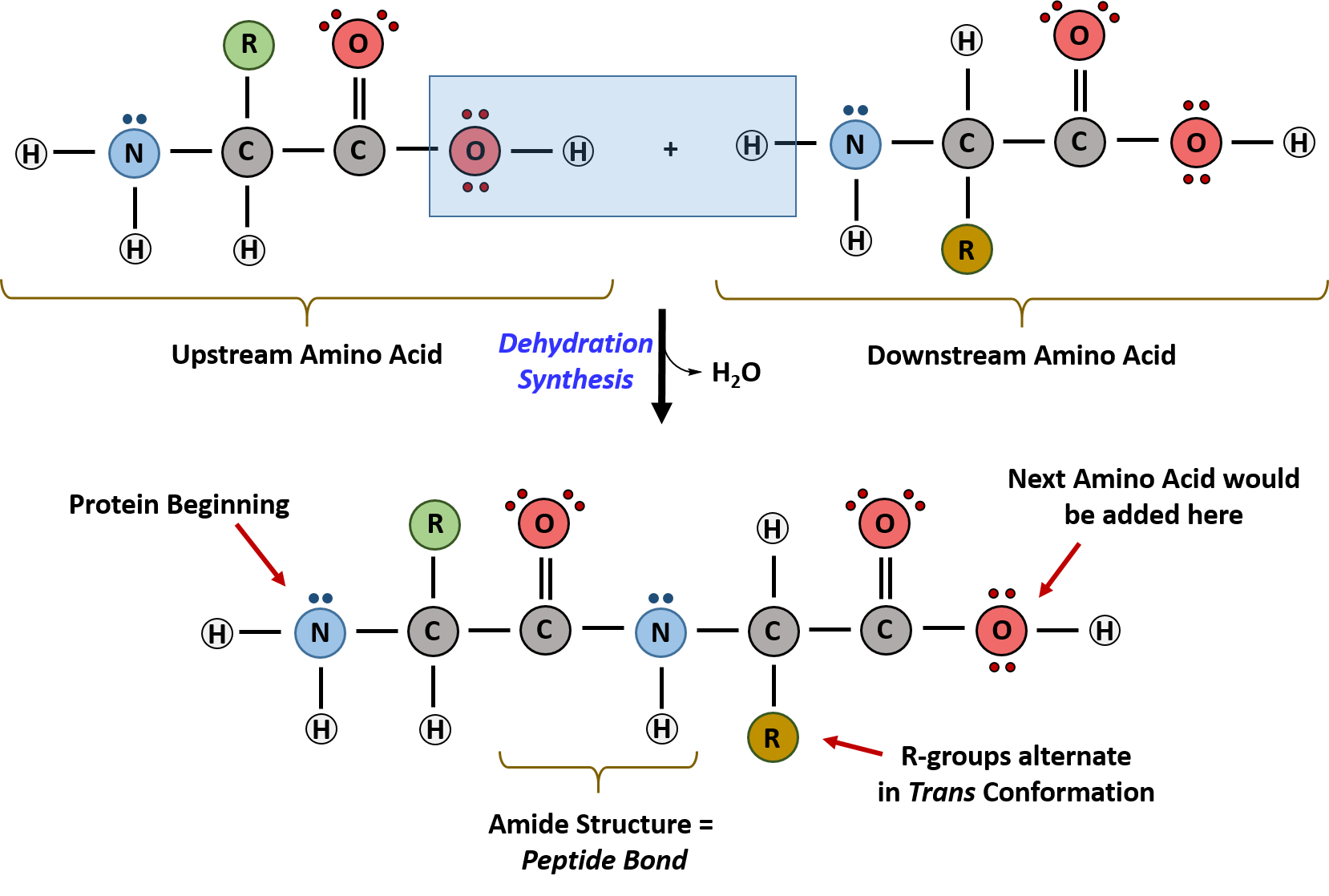
Peptide Bonds form between the amino group of one amino acid and the carboxyl group of another. A single chain of AA held together by peptide bonds is called a polypeptide. London dispersion and hydrogen bonds are present.
Nucleic Acids
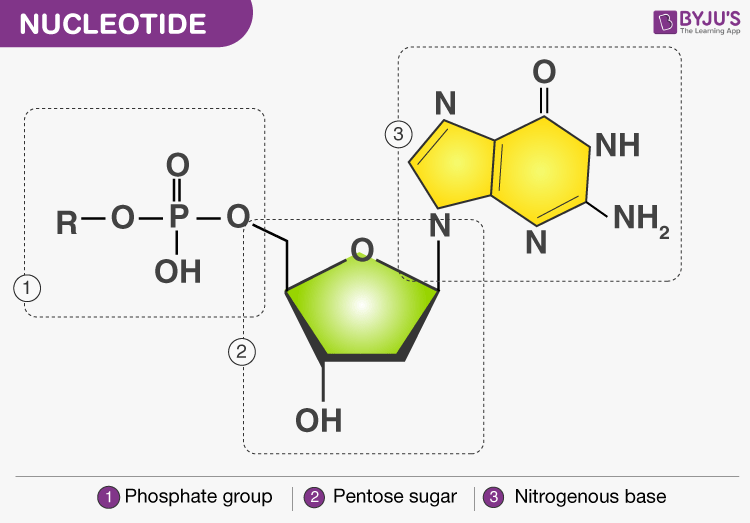
Nucleic Acids are comprised of nucleotide monomers. A polymer of nucleotides is called a "strand".
Nitrogenous DNA bases: Adenine(A), Thymine (T), Guanine (G), Cytosine(C). RNA is the same except T is substitued with Uracil (U).
Nucleotide bases pair together. T and A and G and C are complementary.
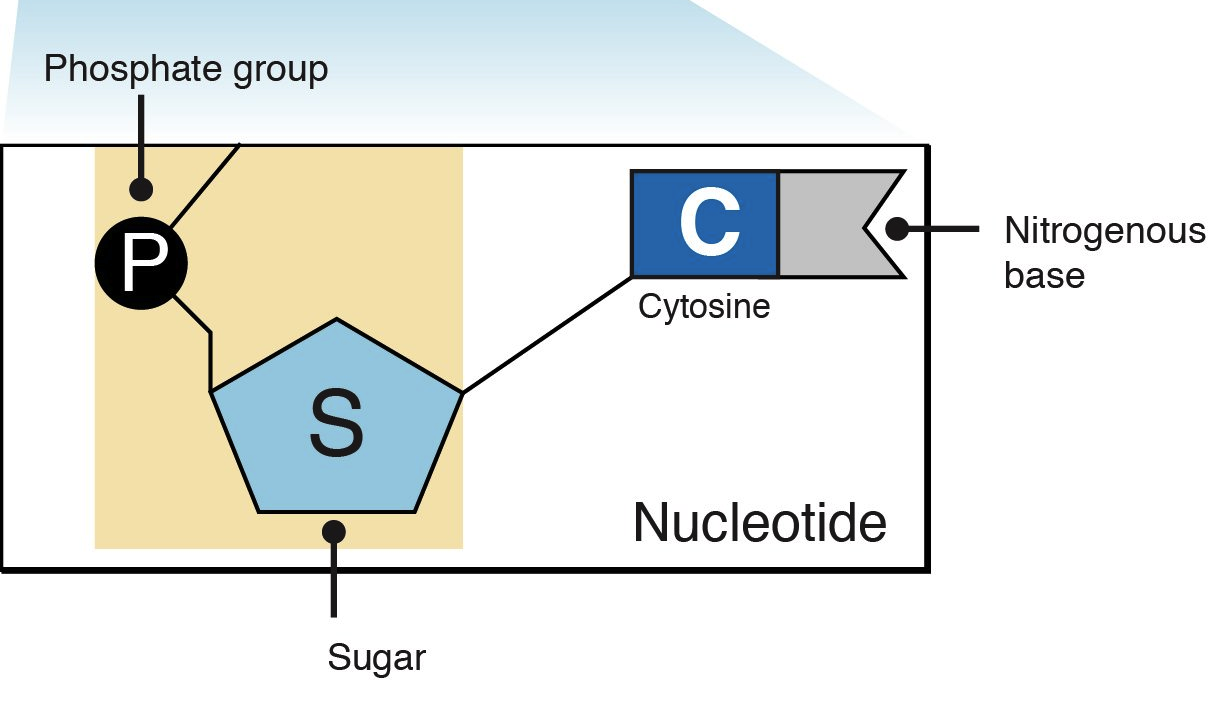
Nucleotide: an organic molecule composed of a sugar bonded to a phosphate group and a nitrogen containing base. (Covalent bonds, phosphate groups, a sugar with 5 carbons, and a nitrogen containing base)

The covalent bond between adjacent strands is called a Phosphodiester bond. The strand is a backbone of alternating phosphate groups and sugars, making them hydrophylic to water. London dispersion is present.

DNA(Deoxyribosnucleic Acid) and RNA(ribonucleic Acid)
Main functions are to store and transfer genetic information and direct sequence and synthesis of new proteins. DNA contains the genetic info of living organisms and is interpreted by the amino acids of the proteins. The amino acid sequence is carried out with RNA molecules. DNA and RNA are polymers made of thousnads of nucleotide monomers.

Condensation Chemical Reaction (Phosphodiester bond)
Lipids
General term for non-polar biological molecules that are mostly comprised of carbon and hydrogen with the lesser extent of oxygen. Lipids are much smaller and less complex than complex carbs and polysaccharides.
They do not dissolve in water and there are 5 main categories of lipids: Fatty acids, fats, phospholipids, steroids and waxes.
Fatty Acids (FAs): The structural backbone and monomers of lipids; a single hydrocarbon chain with a carboxyl group. Usually 14-22 carbons, more results in difficulty with dissolving in water
Saturated FAs have single bonds in the hydrocarbon chain and are solid at room temperature.
Unsaturated FAs have a double bond/s in the hydrocarbon chain and are liquid at room temperature.

Saturated and Unsaturated Fatty Acid Chains
Trans Fatty acids are a type of unsaturated fat that is created by the process of hydrogenation: turning liquid oil into solid fat by adding hydrogen atoms to fill up empty space in an unsaturated FA to imitate a solid, saturated FA. This extends shelf life and can be found in fried foods like doughnuts.

Cis-Fatty Acid vs. Trans Fatty Acid
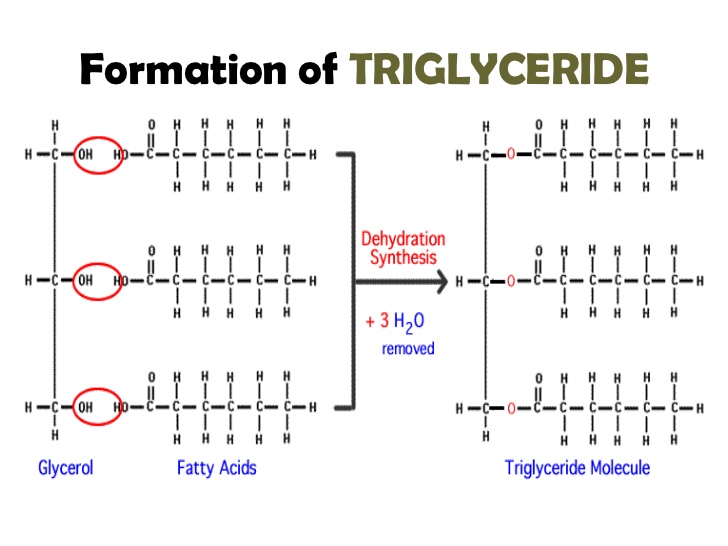
Fats are fatty acids and glycerol; triglyceride, a polymer, can be produced with dehydration synthesis chemical reactions.
These bonds are called ester bonds. Where there is a single bond to a carbon and a double bond to an oxygen, and a singly bonded oxygen to another carbon.
Functions: Fats are common energy storing molecules in living organisms and contain twice the amount of energy as carbs and proteins.
Phospholipids are a lipid with a phosphate group attached. There is a cell membrane called a phospholipid bilayer that responds to temperature changes. Hydrogen bonding is present and london dispersion intermolecular forces are as well.
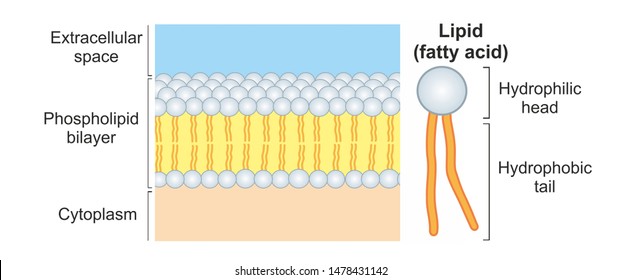
2 hydrophobic FAs and a third site has a charged phosphate group that binds another polar of charged unit. The head is comprised of a phosphate group and glycerol and the tail of 2 fatty acids.

Steroids are a group of lipids with structures with a framework mostly comprised of 4 fused carbon rings. This includes cholesterol and sex hormones like progesterone and testosterone and cholesterol.

Waxes are large lipid molecules comprised of long fatty acid chains linked to alcohols or carbon rings.
Carbohydrates
Functions: Energy storage, structural support, and cell markers
(CH2O)n The most common organic molecule on earth
Carbs contain a high proportion of hydroxyl (OH) groups, are polar molecules, and easily dissolve in water. Sugars and starches store energy in an easily accessible way.

Functional Groups and Monomers and Polymers
Bonds: Glycosidic Linkages are covalent bonds formed between specific hydroxyl (OH) groups. Hydrogen bonds and London dispersionare present. Sugars are polar which means there will be dipole-dipole intermolecular forces as well.
Monosaccharides: single chains of carbons with hydrogen atoms (H) and hydroxyl (OH) groups attached. If the number of carbons is 3-7 the carb is a "simple sugar". An example would be glucose. Other examples are fructose and galactose which are isomers.
Disaccharides: 2 monosaccharides linked with glycosidic linkage. Sucrose is a disaccharide, formed of glucose and fructose.
Oligosaccharides: 2-3 monosaccharides linked via. glycosidic linkages. Example: Sucrose (glucose+ fructose). Like disaccharides but not as complex s poly saccharides.
Polysaccharides: hundreds and thousands of monosaccharides linked with glycosidic linkages. Complex structures composed of monomer glucose. Plants store glucose in the form of starch while animals store glucose in the form of glycogen.
Examples: Cellulose is used for structural support in cell walls and are indigestible by humans as we lack the enzymes to recognize their glycosidic link and break it down. However, starches and glycogen are digestible for humans because we have enzymes that recognize their gly. links and catalyze the cleavage into glucose monomers.

Starches and glycogen differ by the number and types of side chains. More side chains and branches on glycogen molecule= broken down easier for energy than starch.
Isomers: Isomers: One of two or more molecules with the same number and type of atoms but different structural arrangements that result in different functionalities. (Like fructose and galactose arrangements).
Chemical Reaction: Dehydration. In a disaccharide, the hydroxyl group of one mono. bonds with the hydrogen of another mono. and produces a molecule of water and forming a covalent bond. The covalent bond is a glycosidic linkage which can be of the alpha or the beta type (cellulose is beta while glycogen and starch are alpha).

Dehydration of glucose and fructose
