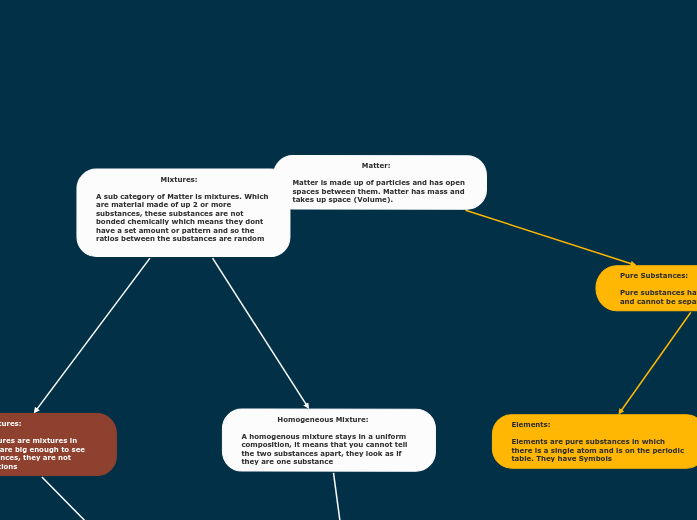
Matter:
Matter is made up of particles and has open spaces between them. Matter has mass and takes up space (Volume).
Mixtures:
A sub category of Matter is mixtures. Which are material made of up 2 or more substances, these substances are not bonded chemically which means they dont have a set amount or pattern and so the ratios between the substances are random
Homogeneous Mixture:
A homogenous mixture stays in a uniform composition, it means that you cannot tell the two substances apart, they look as if they are one substance
Solutions:
Solutions are homogeneous mixtures in which something gets dissolved into something. The substance getting dissolved is called the solute and the substances doing the dissolving is called a solvent
Heterogeneous Mixtures:
Heterogenous mixtures are mixtures in which the particles are big enough to see the different substances, they are not uniformed compositions
Ordinary Mechanical Mixtures:
Ordinary Mechanical Mixtures are Heterogenous mixtures that stay mixed, and so the substances don't go apart from each other
Suspensions:
Suspensions are heterogeneous Mixtures in which the substances will separate from each other when left untouched
Pure Substances:
Pure substances have one type of particle and cannot be separated
Elements:
Elements are pure substances in which there is a single atom and is on the periodic table. They have Symbols
Compounds:
Compounds are the combination of 2 or more elements. They are written in formulas