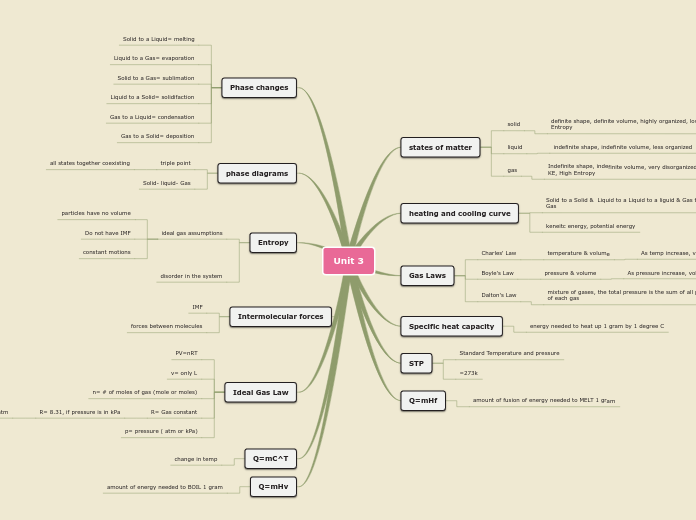
solid
definite shape, definite volume, highly organized, low KE, low Entropy
liquid
indefinite shape, indefinite volume, less organized
gas
Indefinite shape, indefinite volume, very disorganized, High
KE, High Entropy
Solid to a Solid & Liquid to a Liquid to a liguid & Gas to a
Gas
keneitc energy, potential energy
Charles' Law
temperature & volume
As temp increase, volume decrease
Boyle's Law
pressure & volume
As pressure increase, volume decreases
Dalton's Law
mixture of gases, the total pressure is the sum of all pressure
of each gas
energy needed to heat up 1 gram by 1 degree C
Standard Temperature and pressure
=273k
amount of fusion of energy needed to MELT 1 gram
Solid to a Liquid= melting
Liquid to a Gas= evaporation
Solid to a Gas= sublimation
Liquid to a Solid= solidifaction
Gas to a Liquid= condensation
Gas to a Solid= deposition
triple point
all states together coexisting
Solid- liquid- Gas
ideal gas assumptions
particles have no volume
Do not have IMF
constant motions
disorder in the system
IMF
forces between molecules
PV=nRT
v= only L
n= # of moles of gas (mole or moles)
R= Gas constant
R= 8.31, if pressure is in kPa
R= 0.0821, if pressure is in atm
p= pressure ( atm or kPa)
change in temp
amount of energy needed to BOIL 1 gram