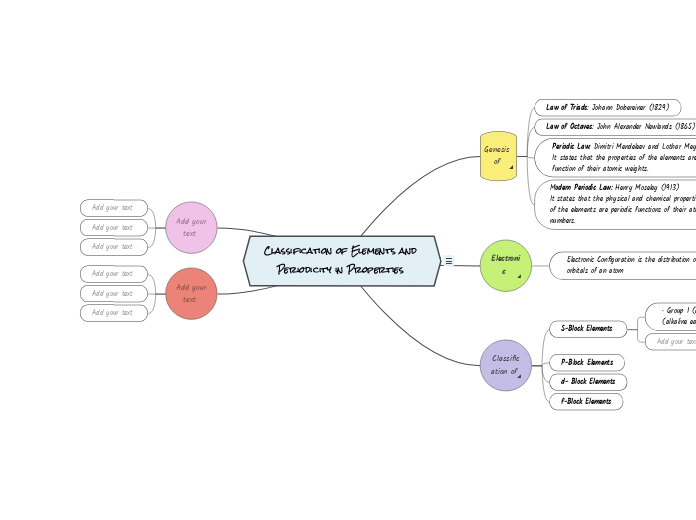
Classification of Elements and Periodicity in Properties
Genesis of Periodic Classification^
Law of Triads: Johann Dobereiner (1829)
Law of Octaves: John Alexander Newlands (1865)
Periodic Law: Dimitri Mendeleev and Lothar Meyer.
It states that the properties of the elements are periodic
function of their atomic weights.
Modern Periodic Law: Henry Moseley (1913)
It states that the physical and chemical properties
of the elements are periodic functions of their atomic
numbers.
Electronic Configuration^
Electronic Configuration is the distribution of electrons into orbitals of an atom
Classification of Periodic table^
S-Block Elements
• Group 1 (alkali metals) and Group 2
(alkaline earth metals)
Add your text
P-Block Elements
d- Block Elements
f-Block Elements
Add your text
Add your text
Add your text
Add your text
Add your text
Add your text
Add your text
Add your text