SNC1D0 Year
Chemistry
Matter
Particle Theory
Different Substances are made up of Different Particles
There is always space between particles
Particles are always moving
Temperature affects the speed that particles move at
Particles are attracted to each other
States of Matter
/three-different-geometric-shapes-blue-red-and-yellow-102115108-58a9a87a3df78c345b430691.jpg)
Solid
Particles are close togther
/waterdrops-splashing-on-water-surface-522937305-582494ed5f9b58d5b15af89b.jpg)
Liquid
Particles are seperated and far from each other

Gas
Particles are very far apart from each other

Plasma
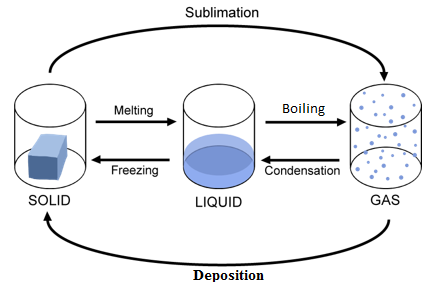
Changes in the State of Matter
Melting
Solid to Liquid
Freezing
Liquid to Solid
Evaporation
Liquid to Gas
Condensation
Gas to Liquid
Sublimation
Solid to Gas
Deposition
Gas to Solid
Pure Substances
Elements
Composed up of only 1 kind of atom
Compounds
Made up of 2 or more different elements in fixed proportions
Only made up of 1 kind of particle
Mixtures
Homogenous
Mixture where the form is uniformed

Heteogenous
Mixture where you can see the different components

Alloys
Properties of Matter
Physical
Description of the substance
Chemical
Describes the ability for a substance to change into another substance
Physical and Chemical Changes
Physical Change
The change in shape, form or state in a substance
Chemical Changes
A change that results in a formation of a new substance
Density
Mass
Amount of matter an object takes up
Volume
Amount of space an object takes up
Amount of matter per unit of volume
Measured in g/mL or g/cm^3
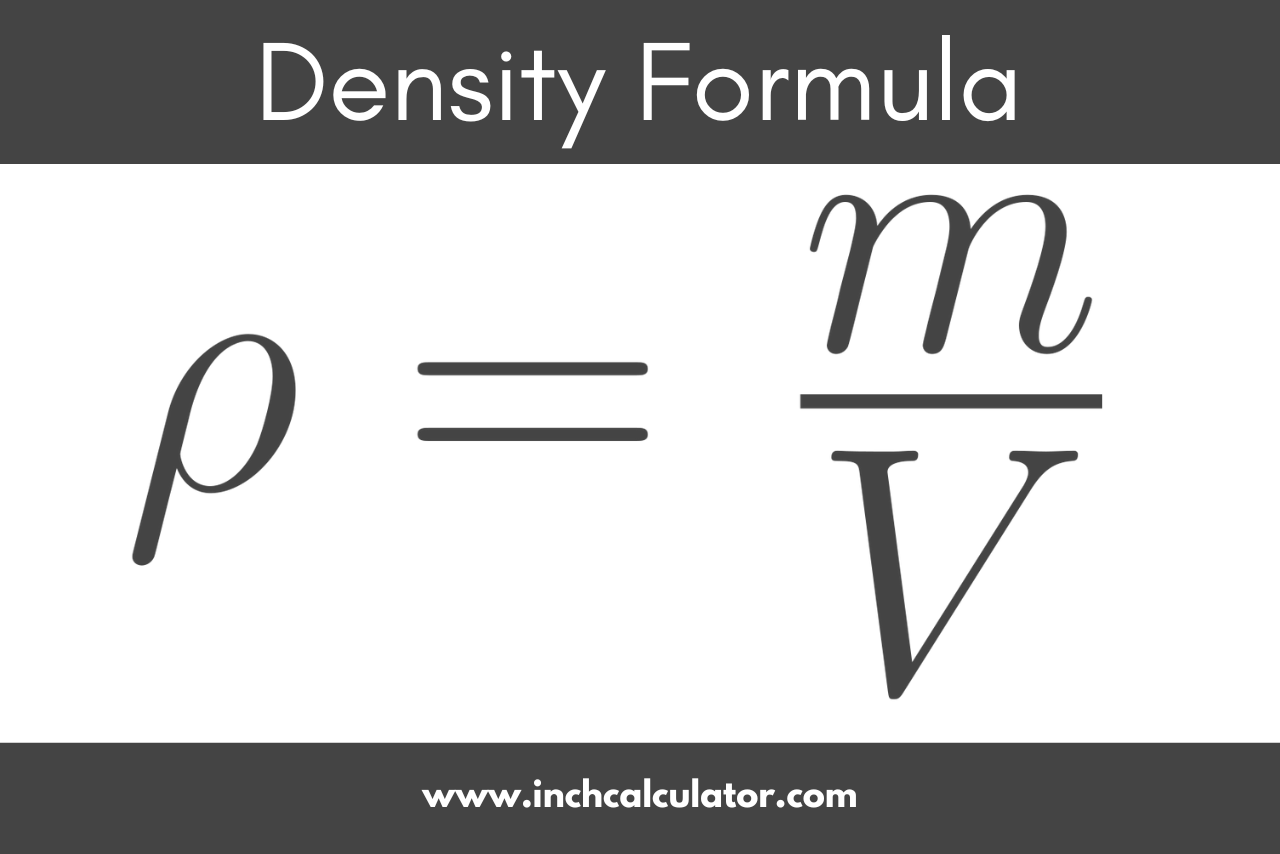
Formula
Atoms
Elements
Compounds

Periodic Table
Rows/Periods
Columns/Families
Hydrogen
Alkali Metals
Group 1
Alkaline Earth Metal
Group 2
Transition Metals
Group 3-12
Halogens
Group 17
Noble Gasses
Group 18
Elements get more reactive the more you go down the columns

Chemical Symbols
Abbreviations of Elements Name
First letter is always a captial
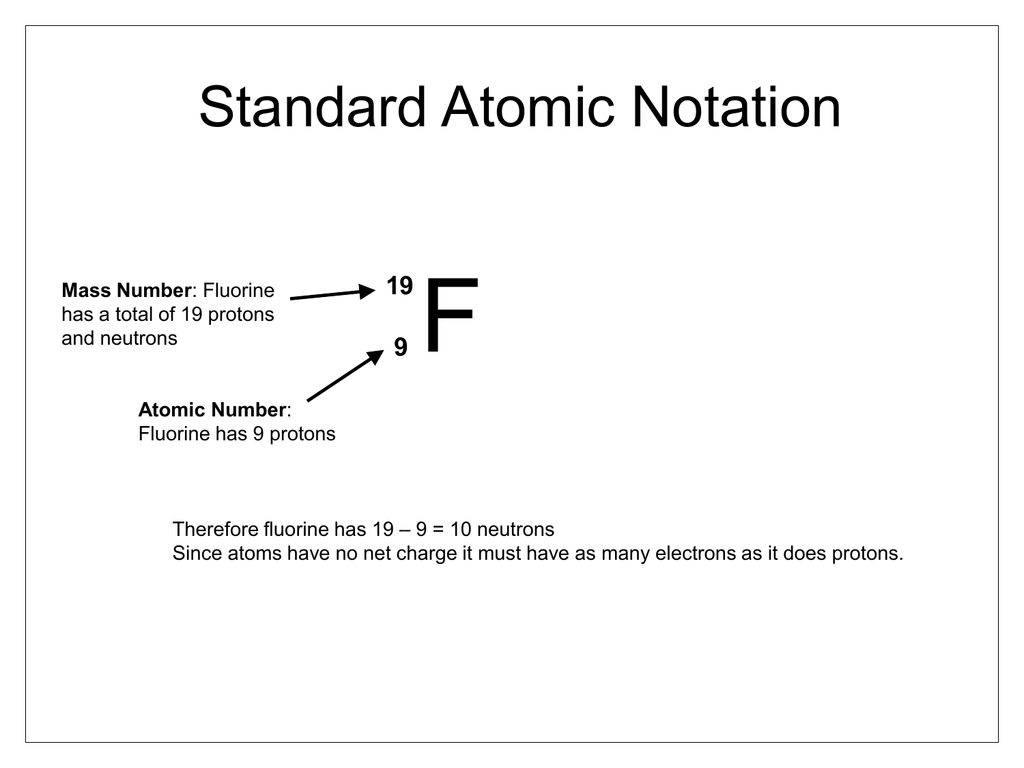
Standard Atomic Notation
Atomic Number
Represents the amount of Protons or Electrons
Atomic Mass
Represents the sum of Protons and Neutrons of the Element

Structure
Proton
Has mass
Positive Charge
Electron
Orbiting the Nucleus
Lies on the "shell"
Shell contains 99% of the Atoms Volume
Shell contains 1% of the Atoms Mass
Have almost no mass
Negative Charge
Neutron
Neutral Charge
Have Mass
Nucleus
Contains the Protons and Neutrons
99% of the Atoms Mass
1% of the Atoms Volume
The Diagrams

Bohr Rutherford Diagram
Relationship between # of electrons every shell
Labels
Dots represent the valence electrons
Valence electrons are electrons on the shell
The p represents the protons in the atom
The n represents the neutrons in the atom
Circle in the middle represents the nucleus
Circles around the middle circle represent the shells
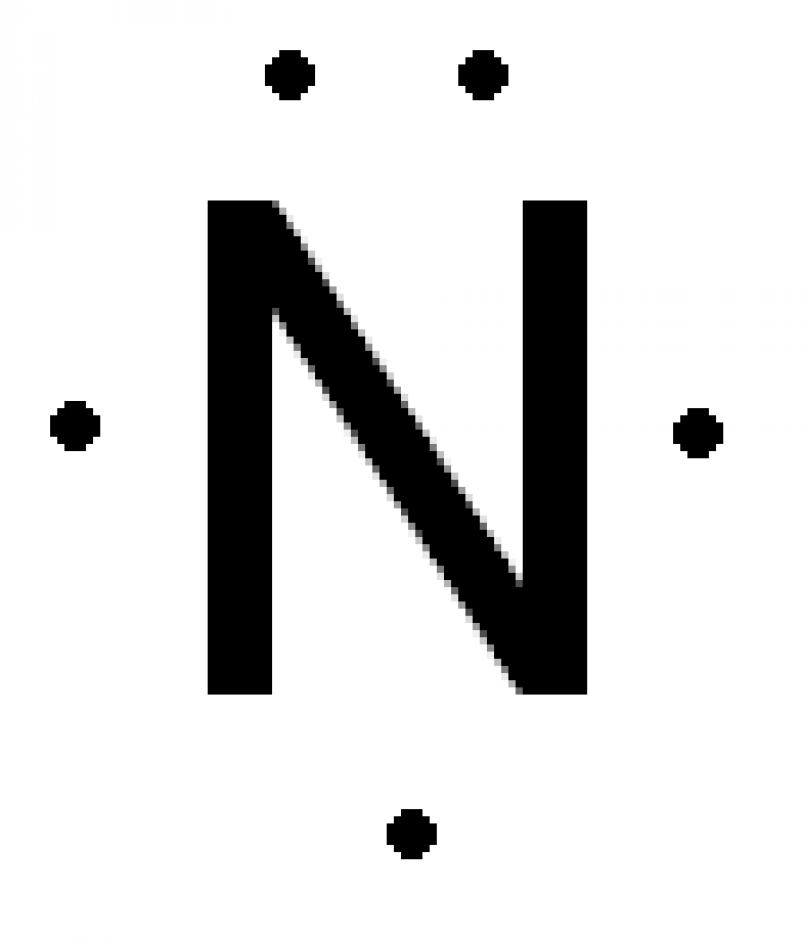
Lewis Dot Diagram
The Letter(s) in the middle are the elements/compounds symbol
The dots surrounding the letter(s) are the valence electrons
Only the electrons on the furthest shell from the middle are represented
Dots are placed singly then placed in pairs in the order: N -> S -> E -> W
A shortcut to finding the valence electrons is looking at the groups the element is in
Starting at 1 electron at group 1, keep increasing by 1 until you reach group 2, where it skips all to way to 3 electrons at group 13. Continue the pattern of 1 electron every group afterwards.
Ions
All atoms want a full outershell to gain stability (2 or 8)
Reason why group 1, which only has 1 valence electron, are the most reactive
Called Stable Octet
Atoms lose or gain more electrons depending to how close they are at the moment to the next full shell
Atoms that gain electrons have a negative charge and are called anions
Atoms that lose electrons have a negative charge and are called cations
Ionic Compounds
Created when a metal and non-metal combine
Metal atoms always lose electrons
Non-metals always gain electrons
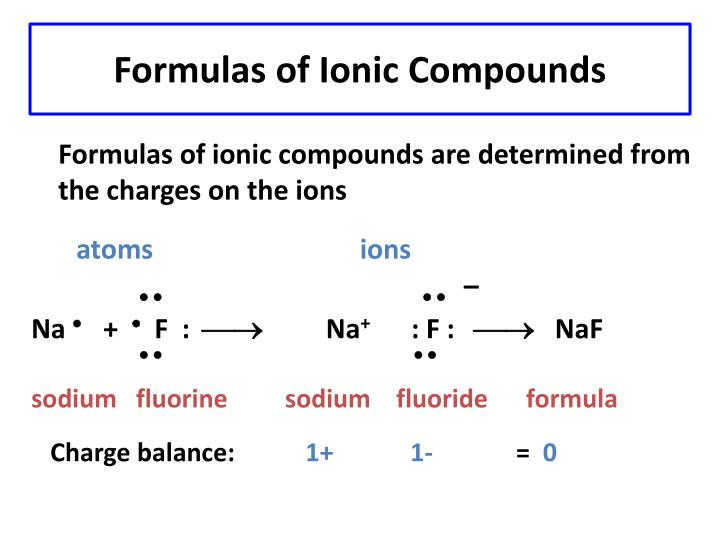
Formula
Steps to making the formula
The first symbol is always the metal followed up by the non-metal
Write the ionic charge above and to the right of the symbol it is attached to
Ionic Charge are the charges that are gained or lost to other elements
Criss cross the charge
Divide the charges to to largest common factor to find the lowest ratio
Counting atoms
The subscripts of a atom represents the amount of the atom there is.
Ex: Cl 2 = 2 Chlorine atoms
Subscripts outside brackets multiply all subscripts within the brackets before it
If there is a coefficient in front of everything, it represents the amount of that element/compound
The coefficient is multiplied with all subscripts within the formula
Alloys
Metals that formed by metaling 2 metals together
Molecular Compounds
A pure substance formed by mixing 2 or more non-metals
Since the nearly have full orbits, they share each others electrons that results in a Covalent Bond

Naming Molecular Compounds
Never add the prefix "mono-" to the first symbol
Always replace the ending of the 2nd symbol with -ide
Chart to show the relationship between # of atoms and the prefixes
Electricity
Static Electricity
Charges
Protons
Cannot Move
Neutrons
Electrons
Can Move
Laws of Charges
Opposites Charges Attract Each Other
Like Charges Repel Each Other
Neutral Objects Attracts All Charges
Neutral Objects are Neutral to Each Other
Insulators
Materials that Prevent the Movement of Electrons

Conductors
Materials that Allow the Flow of Electrons from Atom to Atom

Discharge
Grounding
Connecting a Charged Object to the Ground (The Earth is a Large Conductor that Spreads the Electrons
The Action of Removing a Charge
Charge by Friction
Charge by Contact
Charge by Induction

Electrostatic Series
Current Electricity
Electric Currents
Circuit

Source
Electric Load

Switch/Control
Conductors
Resisitor
Used to reduce current or control voltage
Ohm Meter
Measure Ohm/Resistance
Ammeter
Measures Amps/Current
Voltmeter
Measures Voltage
Motor
Electricity in Real Life Use
Circuit Breakers
Formulas
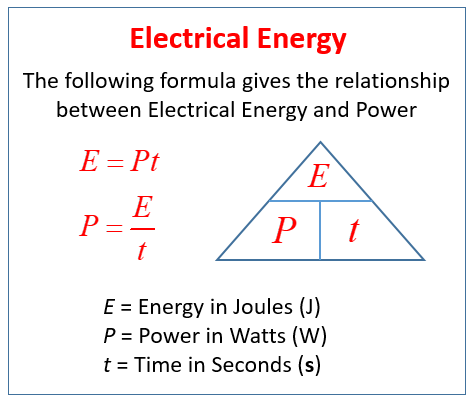
Formula for the Relationship between Power and Electrical Energy
Forumla for the Cost to Operate a Electrical System is: Cost = energy x Price (rate)
Ecology
Environment

Abiotic Factors

Biotic Factors
Climate Change
Ecosystem
Biome
Habitat
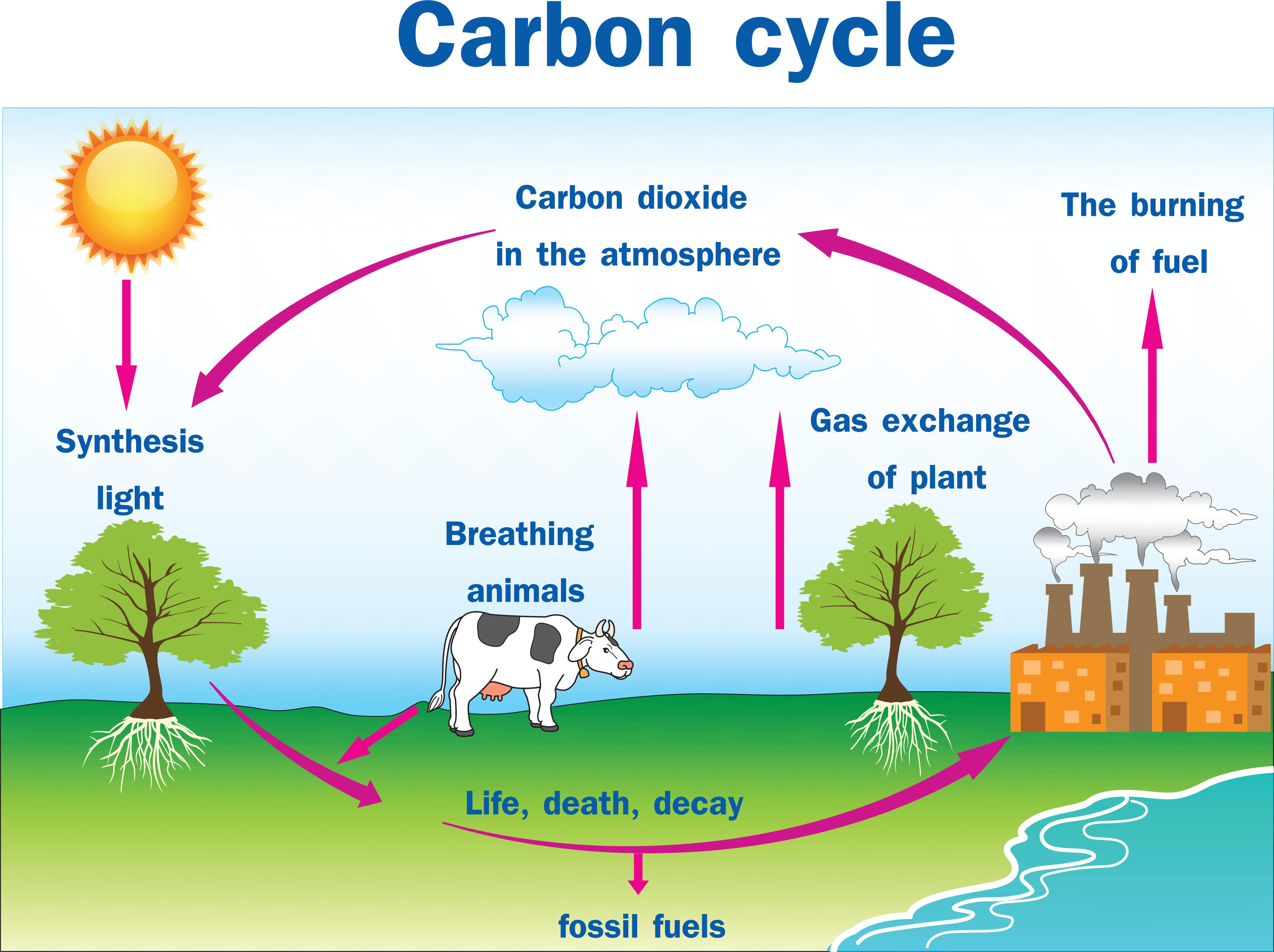
Carbon Cycle
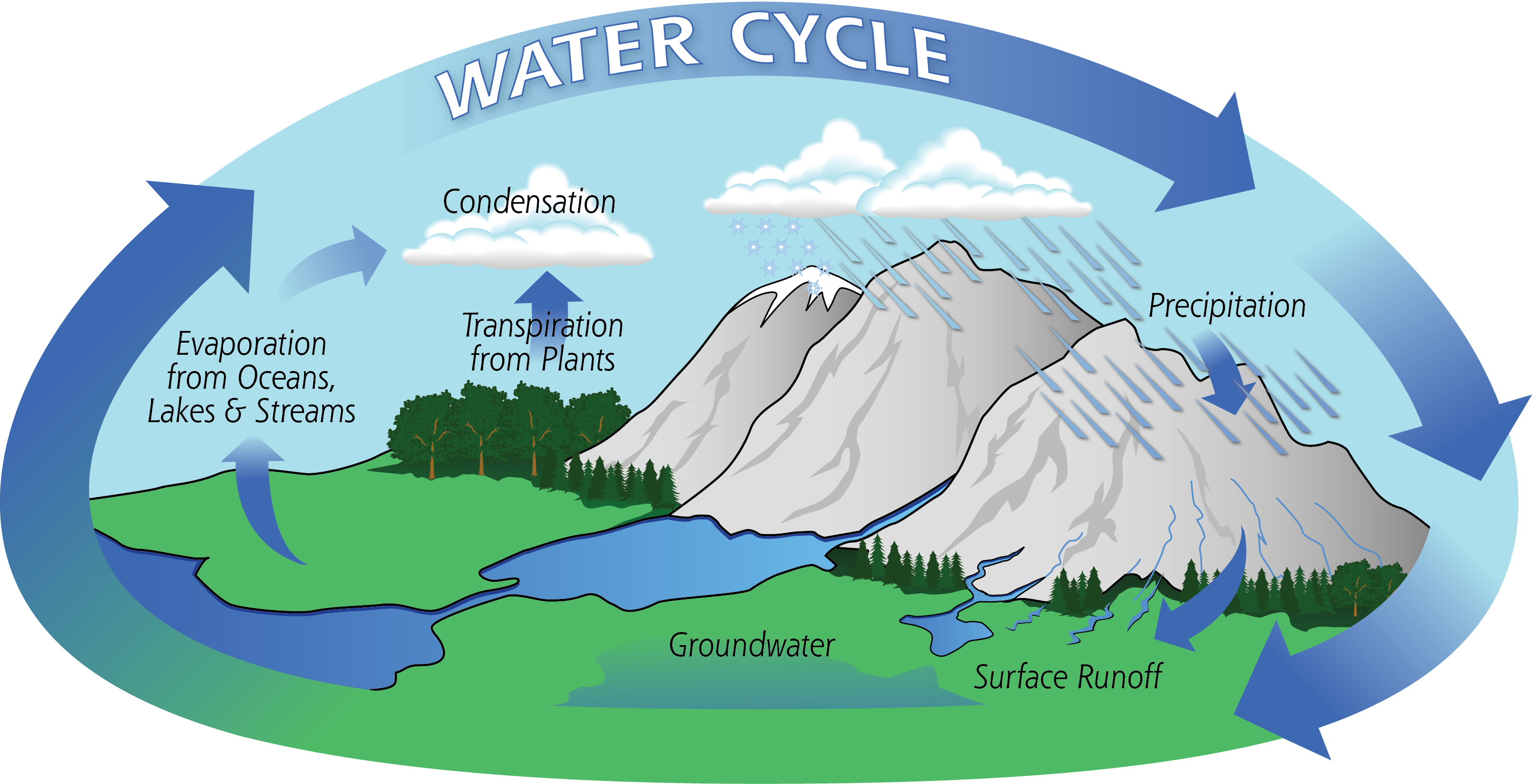
Water Cycle

Nitrogen Cycle
Living Organisms
Species
Population
Communities
Niche
Role of an organism in the environment

Herbivore

Carnivore

Omnivore
Producer/Autotroph
Heterotroph
Prey
Predator

Decomposers/Detritivore
Spheres of the Earth
Biosphere
Lithosphere
Hydrosphere
Atmosphere
Energy Flow

Food Chain

Food Web
Consumer
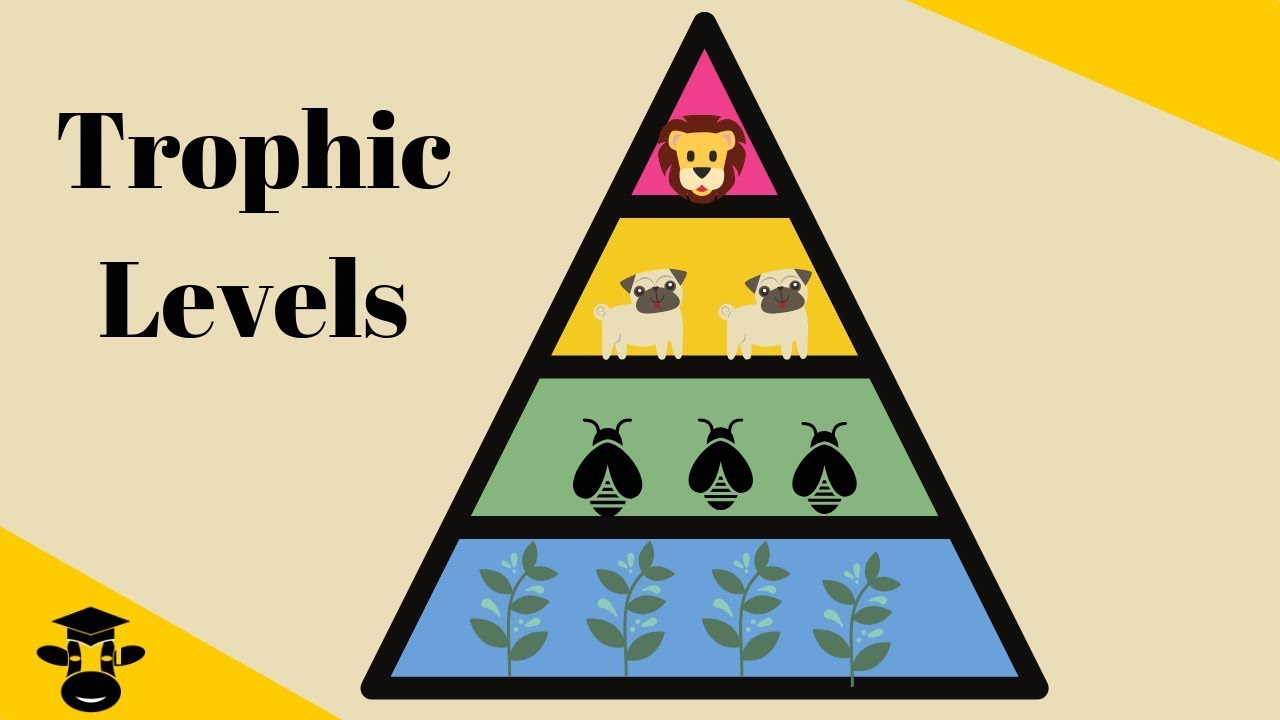
Trophic Levels
Thermal Energy
Light Energy
Photosynthesis
Creates Sugar, Food
Chemical Energy
Cellular Respiration
Creates Co2, Water, Energy using Oxygen and Sugar
Kinetic Energy
Actions
Laws of Thermodynamics
Law of Conservation of Energy
Energy in the universe is constant and cannot be destroyed nor created and can only be transferred
Biogeochemical