Concept map
Substances
Can be
Mixtures
Can contain
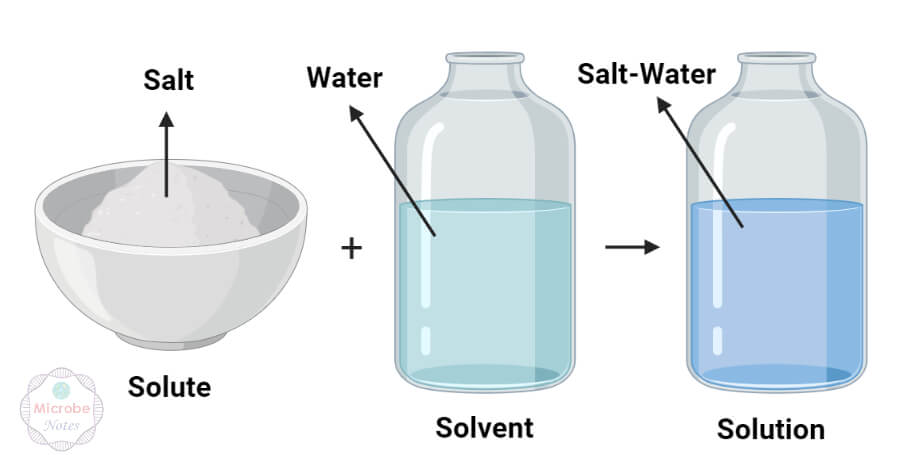
Solvent
water
+
Solute
Salt
Sugar
=
Solution
A Homogenous mixture of substances composed
Such as; Salt Water can be a solution and a Homogenous mixture
Homogenous mixture
A mixture in which the composition is uniform throughout the mixture

Characterized by their
Concentrations
If Known
Standard solution
Molar Solution
Is defined as
An aqueous solution that contains 1 mole (gram-molecular weight) of a compound dissolved in 1 liter of a solution
Percent solution
Is defined as
An amount or volume of chemical or compound per 100 mL of a solution.
Parts Per Million
Is defined as
Unit used for very low concentrations
Heterogeneous mixture
A mixture with a non-uniform composition

Can be
Concentrated
Acids
All react with water to contain H+
Weak

Example
Low concentration of H+ Ions
Strong

Example
High concentration of H+ Ions
Bases
Insoluble
Don't dissolve in water
Soluble
Are called
Alkalis
Sodium Hydroxide, Magnesium Hydroxide
pH Scales
used to measure
electrolyte
In an aqueous solution
A strong electrolyte is considered to be completely ionized, or dissociated, in water, meaning it is soluble.
Non-electrolyte
Does not dissociate, or separate, into ions during the dissolving process.
The separation of ions that occurs when an ionic compound dissolves in water
Dissociation
Neutral
Having no effect on either red
or blue litmus paper
dilute
Stock Solution
A solution that is in stock or on the shelf
Possibly
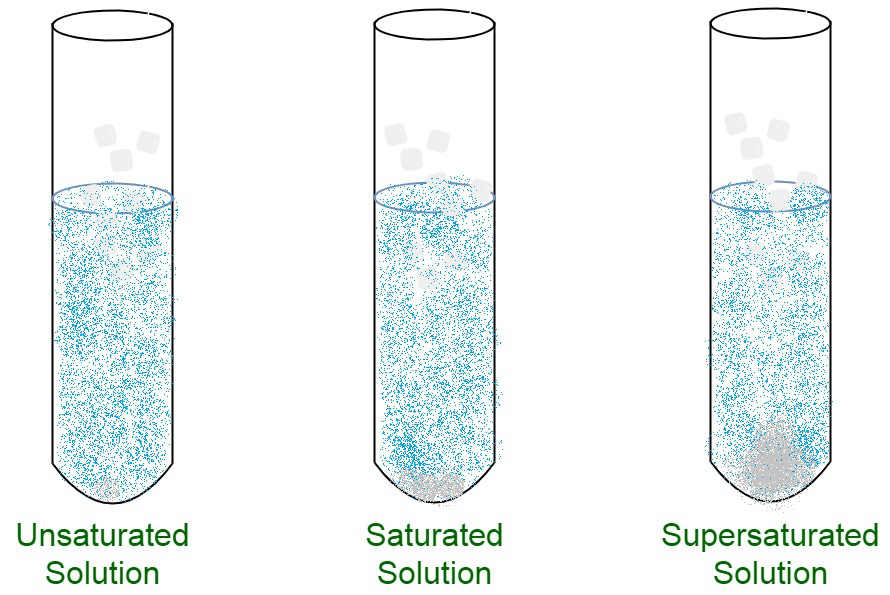
Statured solution
Saturated
Maximum amount of solvent have been dissolved
Supersaturated
A chemical solution
that contains more solute than
the solvent can be hold.
Unsaturated
A solution in which more solute
can be dissolved
molar concentration
The amount of solute, in moles, dissolved in
one liter of solution.
Stock solution
A solution that is in stock or on the shelf
Molecule
Intramolecular forces
Intermolecular
Ionization
Process by which a molecules are
converted to electrically charged
atoms or molecules
Percent concentration
A measure of the amount of solute
dissolved in every 100 units of solution