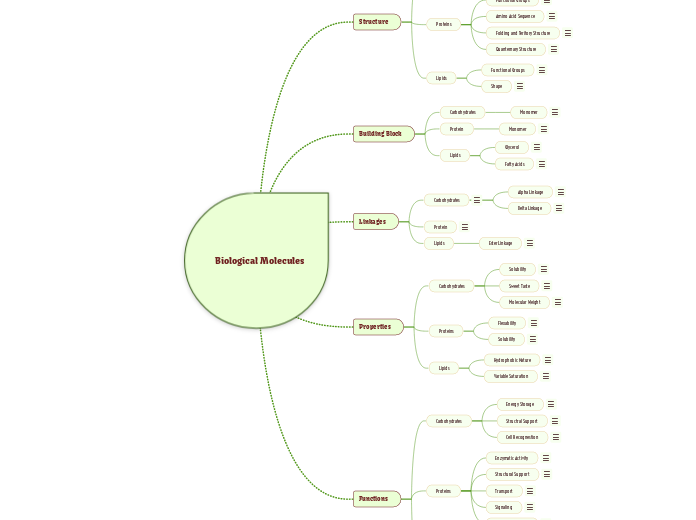
Biological Molecules
Structure
Carbohydrates
Functional Groups
Hydroxyl Group
These groups are characteristics of carbohydrates and are found attached to most carbon atoms in the molecule. They contribute to the hydrophilic nature of carbohydrates, allowing them to form hydrogen bonds with water molecules.
Carbonyl Group
The carbonyl group can be either an aldehyde (-CHO) or a ketone (C=O) group, depending on its position within the carbohydrate molecule. For example, glucose has an aldehyde group, while fructose has a ketone group. The carbonyl group is involved in the reactivity and chemical properties of carbohydrates.
Shape
Carbohydrates can exist in a linear or cyclic (ring) forms. Monosaccharides, such as glucose and fructose, can form cyclic structures in aqueous solutions through intermolecular reactions between the carbonyl group and a hydroxyl group. The ring structure can exist in two main forms: alpha and beta, depending on the orientation of the hydroxyl group attached to the anomeric carbon atom.
Proteins
Functional Groups
Chemical groups like hydroxyl (-OH), amino (-NH2), carboxyl (-COOH), or phosphate (-PO4) determine the chemical properties of proteins. For example, hydroxyl groups can make proteins more polar and hydrophilic, while hydrophobic amino acid side chains can contribute to protein folding and interactions with nonpolar molecules.
Amino Acid Sequence
The sequence of amino acids in a protein, known as its primary structure, is crucial for determining its overall shape and function. Even small changes in the amino acid sequence can have significant effects on protein structure and function.
Folding and Teritory Structure
Proteins fold into specific three-dimensional structures, known as their tertiary structure, which is stabilized by interactions between amino acid side chains. These interactions include hydrogen bonds, ionic bonds, disulfide bonds, and hydrophobic interactions. The tertiary structure determines the protein's overall shape and its ability to perform specific functions.
Quanternary Structure
Some proteins consist of multiple polypeptide subunits, each with its own tertiary structure, which assemble into a larger functional protein complex. The arrangement and interactions between these subunits contribute to the protein's quaternary structure and overall function.
Lipids
Functional Groups
Lipids consist predominantly of hydrocarbon chains, often with a carboxyl group (COOH). This gives them their characteristic hydrophobic nature.
Shape
The shape of lipids varies based on their molecular structure. For example, triglycerides have a glycerol backbone attached to three fatty acid chains, while phospholipids have a phosphate group attached to a glycerol backbone along with two fatty acid chains.
Building Block
Carbohydrates
Monomer
A monomer is the basic building block or unit from which larger molecules, called polymers, are constructed. In the context of carbohydrates, monomers refer to single sugar molecules.Carbohydrates are composed of monosaccharides, which are the simplest form of sugars. These monosaccharides consist of a single sugar molecule and cannot be further hydrolyzed into simpler compounds.Monosaccharides typically have the general chemical formula (CH2O)n, where "n" represents the number of carbon atoms. They contain functional groups as hydroxyl (-OH) groups and a carbonyl (C=O) group, which determine their chemical properties and reactivity.Examples of monosaccharides include glucose, fructose, and galactose. Each of these monosaccharides has a unique arrangement of atoms, resulting in distinct chemical and physical properties.Monosaccharides serve as the building blocks for larger carbohydrates, such as disaccharide, oligosaccharide, and polysaccharides. Through condensation reactions, monosaccharides can join together to form glycosidic bonds, linking multiple monomers and creating complex carbohydrate structures.The type of monosaccharide present in a carbohydrate molecule influences its properties and functions. For example, glucose is a primary source of energy for cellular processes, while fructose is sweeter in taste and commonly found in fruits.
Protein
Monomer
Proteins are polymers made up of monomer units called amino acids. Amino acids consist of a central carbon atom bonded to a hydrogen atom, an amino group (-NH2), a carboxyl group (-COOH), and a side chain (R group) that varies among different amino acids. There are 20 different amino acids commonly found in proteins, each with its own unique side chain. The sequence of amino acids in a protein determines its primary structure, which in turn influences its folding and overall three-dimensional structure.
Lipids
Glycerol
A three-carbon alcohol that serves as the backbone for triglycerides and phospholipids. It provides the foundation for lipid molecules to attach fatty acid chains.
Fatty Acids
Chains of hydrocarbons with a carboxyl group (COOH) at one end. They can vary in length and saturation, influencing the properties and functions of lipids.
Linkages
Carbohydrates
Glycosidic bonds are covalent bonds that join two monosaccharides together through a condensation reaction, also known as dehydration synthesis.During this reaction, a hydroxyl (-OH) group from one monosaccharide combines with a hydrogen atom from the hydroxyl group of another monosaccharide, resulting in the formation of a covalent bond and the release of a water molecule (H2O).The bond formed between the two monosaccharides is called a glycosidic bond, and it links the anomeric carbon atom of one monosaccharide to a hydroxyl group on another monosaccharide.
Alpha Linkage
In alpha linkages, the hydroxyl group attached to the anomeric carbon atom of one monosaccharide points downward relative to the plane of the sugar ring structure. Alpha linkages are often found in storage polysaccharides like glycogen and starch.
Delta Linkage
In beta linkages, the hydroxyl group attached to the anomeric carbon atom of one monosaccharide points upward relative to the plane of the sugar ring structure. Beta linkages are commonly found in structural polysaccharides like cellulose.
Protein
Amino acids are linked together through peptide bonds, formed by dehydration synthesis between the carboxyl group of one amino acid and the amino group of another amino acid. During peptide bond formation, the carboxyl group of one amino acid undergoes a condensation reaction with the amino group of another amino acid, resulting in the release of a water molecule and the formation of a covalent peptide bond. This process repeats sequentially, forming a linear chain of amino acids known as a polypeptide.
Lipids
Ester Linkage
Fatty acids are attached to the glycerol backbone through ester linkages, formed through dehydration synthesis. This linkage is key to the structure of triglycerides and phospholipids.
Properties
Carbohydrates
Solubility
Hydrophilic Nature: Carbohydrates, particular monosaccharides and smaller carbohydrates, are generally soluble in water due to their hydrophilic nature. This is because carbohydrates contain multiple hydroxyl (-OH) groups, which can form hydrogen bonds with water molecules.Effect on size: smaller carbohydrates, such as monosaccharides and some disaccharides, have high solubility in water because their small size allows them to interact effectively with water molecules. However, larger polysaccharides, such as cellulose and some starches, may have limited solubility in water due to their larger molecular size and complex structures.
Sweet Taste
Molecular Structure: Some carbohydrates, such as glucose, fructose, and sucrose, have a sweet taste due to their specific molecular structures and the way they interact with taste receptors on the tongue. Monosaccharides: Monosaccharides like glucose and fructose are known for their sweet taste. Glucose is commonly found in fruits and honey, while fructose is naturally occurring in fruits, honey and some vegetables.Disaccharides: Disaccharides like sucrose (table sugar), which is composed of glucose and fructose, also have a sweet taste.
Molecular Weight
Varied Molecular Weight: Carbohydrates exhibit a wide range of molecular weights depending on the number of monosaccharide units they contain.Monosaccharides: Monosaccharides are the smallest carbohydrates and have relatively low molecular weights. For example, glucose has a molecular weight of approximately 180 g/mol.Polysaccharides: Polysaccharides, such as starch and glycogen, consist of multiple monosaccharide units linked together. As a result, they have higher molecular weights compared to monosaccharides. For example, starches can have molecular weights ranging from a few thousand to several million Daltons, depending on their degree of polymerization.
Proteins
Flexability
Proteins can exhibit flexibility or conformational changes, allowing them to adapt to different environments or interact with other molecules. Conformational changes are often essential for proteins to perform their functions effectively.
Solubility
Solubility of proteins varies depending on the amino acid composition and the environment. Some proteins are soluble in water (hydrophilic), while others are insoluble in water (hydrophobic), often due to the presence of hydrophobic amino acid side chains.
Lipids
Hydrophobic Nature
Lipids are nonpolar molecules due to their predominantly hydrocarbon structure, making them insoluble in water but soluble in nonpolar solvents.
Variable Saturation
Fatty acids can be saturated (no double bonds), monounsaturated (one double bond), or polyunsaturated (multiple double bonds), affecting the fluidity and stability of lipid membranes.
Functions
Carbohydrates
Energy Storage
Carbohydrates serve as an essential energy source for living organisms. Starch, found in plants, and glycogen, found in animals, are polysaccharides used for storing energy.
Structral Support
Certain carbohydrates provide structural support to cells and tissues. For example, cellulose, a polysaccharide found in plant cell walls, provides rigidity and strength to the cell wall structure.
Cell Recognestion
Carbohydrates are involved in cell recognition and communication. Glycoproteins and glycolipids, which are proteins and lipids with attached carbohydrate chains, play crucial roles in cell signaling and recognition processes on cell membranes.
Proteins
Enzymatic Activity
Proteins called enzymes catalyze biochemical reactions by binding to specific substrates and facilitating their conversion into products. Examples include digestive enzymes like amylase, which breaks down carbohydrates, and DNA polymerase, which synthesizes DNA molecules during DNA replication.
Structural Support
Structural proteins like collagen and keratin provide support and stability to cells and tissues by forming fibers or networks. For example, collagen forms the structural framework of connective tissues like tendons, ligaments, and skin, while keratin makes up the structure of hair, nails, and the outer layer of skin.
Transport
Transport proteins, such as ion channels and carrier proteins, facilitate the movement of molecules across cellular membranes. For instance, ion channels allow ions like sodium (Na+) and potassium (K+) to move across cell membranes, while carrier proteins transport molecules like glucose and amino acids into cells.
Signaling
Signaling proteins, such as receptors, transmit signals within cells or between cells, initiating cellular responses to external stimuli. For example, G protein-coupled receptors (GPCRs) respond to signaling molecules like hormones and neurotransmitters, initiating intracellular signaling cascades that regulate various cellular processes.
Immune Response
Proteins of the immune system, like antibodies, recognize and bind to foreign molecules (antigens), facilitating their removal from the body. Additionally, proteins like cytokines regulate immune responses by signaling between immune cells and coordinating the body's defense against pathogens.
Lipids
Energy Storage
Triglycerides
Act as the primary storage form of energy in adipose tissue, providing a concentrated source of metabolic fuel.
Structural Role
Phospholipids
Form the structural basis of cell membranes, with hydrophobic tails facing inward and hydrophilic heads facing outward, creating a semi-permeable lipid bilayer.
Insulation
Adipose tissue, composed largely of triglycerides, serves as an insulating layer beneath the skin, helping to maintain body temperature.
Protection
Phospholipids in cell membranes provide a protective barrier, shielding the cell's internal components from the external environment and regulating the passage of molecules.
Signaling
Steroids, a type of lipid, serve as signaling molecules that regulate various physiological processes, such as hormone signaling and gene expression.