Chemistry
Periodic Table
Location of each element
Bohr-Rutherford Diagram
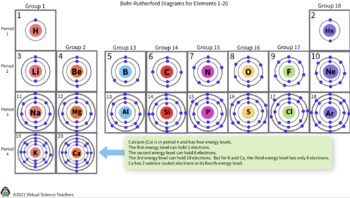
The number of e- are shown using dots in shells around the nucleus, each element is organized in the periodic table according to the number of dots electrons it holds and the number of rings. Left - Rights: increases electrons. Top - Bottom: Increases number or rings.
Ionic Charge
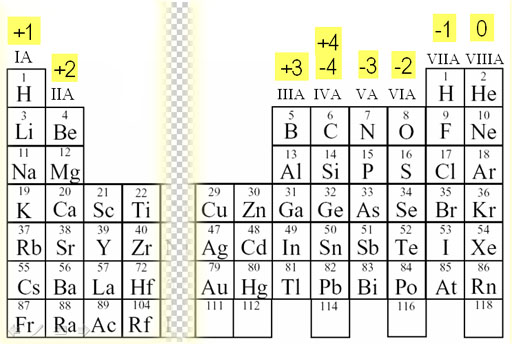
The charges are lower on the outer ends of the table and the charges are the highest near the center.
Formulas
Ionic Compounds - First write the symbols for each and write the charges above them, then crisscross the numbers and put them below the symbols to both elements. If both numbers are the same then its not necessary to write numbers or crisscross
Example in Chemical Form for Ionic Compounds - Mg2+ + I- : MgI2
Multivalent Compounds - In word form for these compounds the charges would be presented with the numerals, you transfer them into numbers and then do the same thing you do with ionic compounds and crisscross them to get the correct formula
Compounds containing polyatomic ions - For these types of compounds, it is important to balance out electrons so that numbers match up.
Diatomic Elements
Ex. Hydrogen (H), Nitrogen (N), Oxygen (O), Fluorine (F), Chlorine (Cl), Bromine (Br), and Iodine (I).
Elements that have molecule composed of two of the same atom.
Difference between Formulas for Ionic compounds and Molecular compounds
Ionic - A formula that contains a non-metal AND a metal such as NACI
Molecular ( Covalent ) - A formula of elements that are only metals such as H2O
Materials
General Characteristics of Acids and Bases
Bases - Tastes bitter, 7-14 on the PH scale, have hydroxide ions in their formulas, when dissolved in water the OH- ions make the product basic, exact opposite of acids
Acids - Tastes sour, 1-6 on the PH scale, they are corrosive, example of common acids : HCI, HF, H2SO4, etc.
Chemical Changes
Change in colour
Formation of Gas
Change in Odor
Change in temperature
Light is being produced
Something starts to burn
Balancing equations
Predicting the product of chemical reactions given materials
In order to determine the product, when you start with materials you must balance them out for every element to contain the same amount of ions to predict the correct product. (reaction + reaction = product). So adding ions and multiplying one side will need to happen for all the same ions to be the same number. In the instance above, you must get both Fe's to be the same along with O and C to be that same number.
Compounds
Simple Ionic Compounds - Sodium Chloride, Potassium Ionide, Magnesium Oxide
Multi-Valent Metal Compounds - Iron (II) Chloride, Copper(III) Sulfate, Lead (IV) Nitrate
COmpounds Containing Polyatomic Ions - Sodium Bicarbonate, Calcium Sulfate, Ammonium Nitrate
Reactions
Synthesis Reaction - two or more reactants combine to make a product. Ex: 2 H2 + 02 = 2 H20
Decomposition Reaction - When a single compound ( product) is broken down into two or more substances. Ex: 2 H2O = 2 H2 + 02
Single Displacement Reaction - One element replaces another in a compound which then produces a new compound.
Combustion Reaction - When a substance reacts with oxygen creating heat or light. Ex: hydrocarbon + 02 = CO2 + H2O
Elements
Cation - Are formed when a metal looses its electrons. If they end up with fewer electrons then protons, they are they then have a positive charge
Anion - they are formed when a non-metal gains electrons making them have a full octet. When this happens they are then a negative ion.
Difference between elements, ionic compounds and molecular compounds.
Elements - Compounds are elements that are bonded together and elements are singular.
Ionic Compounds - Composed with positive and negative ions (cations and anions) and are held together by ionic bonds.
Molecular Compounds - Compounds made up of non - metal that are bonded to each other.
Naming Molecular Compounds
Prefixes are used to dictate the number of a given element present in the compound.
“Mono-” indicates one, “di-” indicates two, “tri-” is three, “tetra-” is four, “penta-” is five, “hexa-” is six, “hepta-” is seven, “octo-” is eight, “nona-” is nine, and “deca” is ten.
Laws of Conservation of Mass
The Mass of the system is the same before and after the reaction and the number of atoms does not change. it is not created or destroyed by chemical reactions or physical changes. Just like the mass stays the same when ice becomes water.
This goes for as long as it is a CLOSED SYSTEM
PH Scale
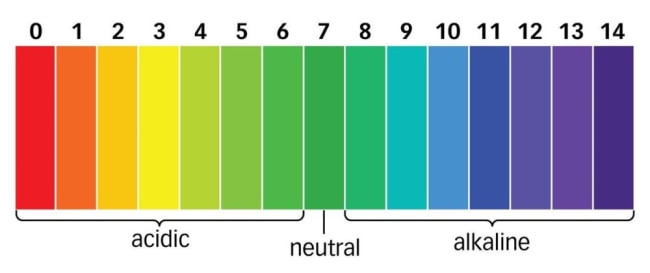
PH scales determine how acidic or basic water is. it measures the relative amount of free hydrogen and hydroxyl that is in the water. You can determine this by the colour the water changes, if very purple you know it is basic, if green, neutral and if red, acidic. This scale is used when chocking hot tubs or pools to know what it needed to have clean and safe water.
Equations
Differences between different types of equations
Balancing Equations - adjusted so that the law of conservation is upheld, they include the most details with coefficents to represent quantities of reactants and products. The key like i said before is to have the same numbers of atoms when working with these types of equations
Word equations - this form describes reactions using words rather then chemical formulas
Skeleton Equations - provides basic outlines of the reaction, they show how many atoms are in each element and they demonstrate the product for compounds when two elemetns are added together. For example, H2 +O2 = H2O
Activity Series
Elements with a higher rank in the series are more reactive in a single replacement reaction. So therefore a single replacement will occur when a element that is less reactive can be displaced by an element that is higher up on the rankings.