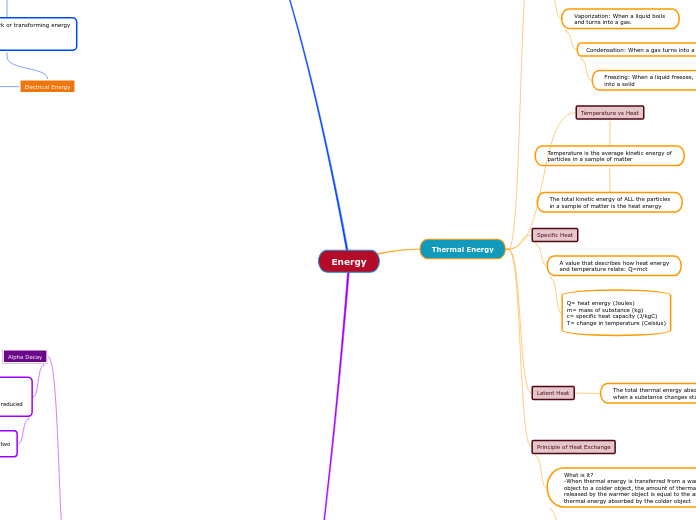
Changes of State
Fusion: When a solid melts
and turns into a liquid
Vaporization: When a liquid boils
and turns into a gas.
Condensation: When a gas turns into a liquid
Freezing: When a liquid freezes, turning
into a solid
Temperature vs Heat
Temperature is the average kinetic energy of
particles in a sample of matter
The total kinetic energy of ALL the particles
in a sample of matter is the heat energy
Specific Heat
A value that describes how heat energy
and temperature relate: Q=mct
Q= heat energy (Joules)
m= mass of substance (kg)
c= specific heat capacity (J/kgC)
T= change in temperature (Celsius)
Latent Heat
The total thermal energy absorbed/released
when a substance changes state (Q)
Latent Heat of Fusion (Q=mLf):
-The amount of thermal energy required
to change a solid into a liquid or a liquid
into a solid
Latent Heat of Vaporization (Q=mLv):
-The amount of thermal energy required
to change a liquid into a gas or a gas into
a liquid
Principle of Heat Exchange
What is it?
-When thermal energy is transferred from a warmer
object to a colder object, the amount of thermal energy
released by the warmer object is equal to the amount of
thermal energy absorbed by the colder object
Qreleased + Qabsorbed=0
m1 c1 T1 + m2 c2 T2=0
Potential Energy
The ability of an object to do work because of forces
in its environment
Gravitational Potential Energy: energy stored
due to the height at which force of gravity can act. Energy
possessed by objects that are affected by the force of gravity
Eg=mgh
Kinetic Energy
The energy possessed by moving objects
Kinetic Energy is calculated using an objects mass and velocity: Ek=mv^2/2
Work
Work is done whenever a force causes motion or
a change in motion
-W=Force x displacement
-Scalar quantity
-Unit is Joule
-If the force applied is 0, no work is done
Efficiency
The ratio of the useful energy provided by a device
to the energy required to operate the device
efficiency= Eout/Ein x100%
Power
The rate of doing work or transforming energy
P=W/t
Unit is watt (W)
1HP=750W
Electrical Energy
E=Power x time
Types of Radioactive Emissions
Alpha Decay
-A nuclear reaction in which an alpha particle
is emitted.
-When a substance undergoes alpha decay, the mass
number is reduced by four and the atomic number is reduced by two
Alpha particle: a particle emitted during alpha decay composed of a helium nucleus containing two protons and two neutrons
Beta Decay
Nuclear reaction in which a beta particle is emitted/captured
Beta particle: a high energy electron or positron ejected/captured by a nucleus during beta decay
Beta Positive Decay
-A proton changes into a neutron and positron
-the mass number remains unchanged but the atomic
number decreases by one
Beta Negative Decay
-When the nucleus contains too many neutrons, the
strong nuclear force becomes greater than the electrostatic force
-the mass number remains unchanged but the atomic number
increases by one
Electron Capture
-Electron is absorbed by a nucleus and combines with a
proton to form a neutron
-The mass number remains unchanged but the atomic number
decreases by one
Gamma Decay
A reaction in which an excited nucleus returns to a
lower, more stable energy state, releasing a very
high-energy gamma ray in the process
Gamma ray: very high-energy electromagnetic radiation
Excited State: The * indicates that the parent in this reaction
is in the excited state
Half-Life
The time it takes for half the atoms of a radioactive
substance to decay
A=Ao (1/2)^t/h
A=mass of radioactive material at time t
Ao= initial mass of radioactive material
t= time elapsed
h= half-life of the radioactive material
Fission and Fusion
Nuclear Fission: occurs when a large, unstable nucleus
breaks apart into smaller, more stable nuclei (large amounts
of energy are produced by the fission process)
Nuclear Fusion: a nuclear reaction whereby two light
atomic nuclei fuse/combine to form a single larger, heavier nucleus
Law of Conservation of Mass-Energy
E=mc^2
Where E is the energy released (MeV)
m is the mass defect (u)
c^2 is 930 MeV/u