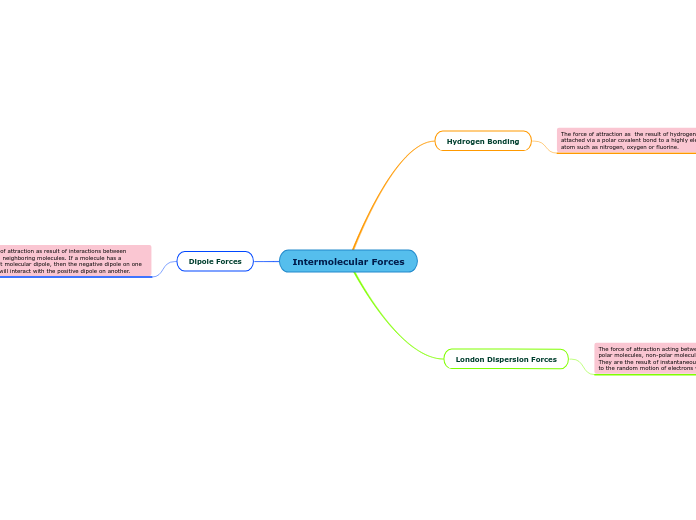
The force of attraction as the result of hydrogen being attached via a polar covalent bond to a highly electronegative atom such as nitrogen, oxygen or fluorine.
Strongest of three intermolecular forces
Hydrogen is attracted to lone pair of electrons on neighboring molecule
Nylon, a polyamide forms hydrogen bonds with nitrogens
The force of attraction acting between all entities, including polar molecules, non-polar molecules and unbonded atoms. They are the result of instantaneous dipoles being created due to the random motion of electrons within a molecule.
Weakest Intermolecular Force
Occur in all molecule types
Larger Molecule= Greater Polarizability= Stronger IMF
Ex. Stretching plastic bag breaks dispersion forces and straightens polyethylene chains
The force of attraction as result of interactions between dipoles on neighboring molecules. If a molecule has a permanent molecular dipole, then the negative dipole on one molecule will interact with the positive dipole on another.
Increased polarity= Increased in force
Dipole-Dipole, Ion-Dipole, Induced Dipole-Dipole
Stronger than london forces, weaker than hydrogen bonding
PVC has many induced dipole-dipole interactions, making it hard and brittle before addition of plasticizer