von Mariana Trillos Vor 7 Monaten
168
Regulation of Blood pH in the Human Body
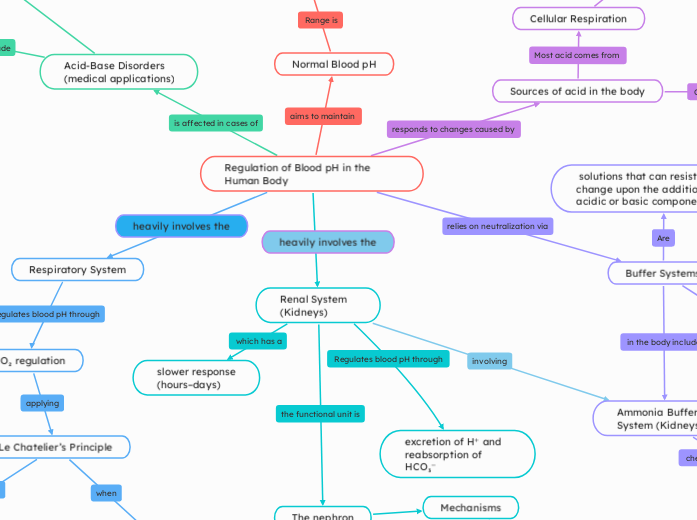
von Mariana Trillos Vor 7 Monaten
168

Mehr dazu
high HCO₃⁻ or low acid
low HCO₃⁻ or high acid
Collecting Duct
traps H⁺ using NH₃ →NH₄⁺ (pee)
Distal tubule
Proximal tubule
metabolizes glutamine → 2 NH₄⁺ + 2 HCO₃⁻
Secrete H⁺
Reabsorb HCO₃
CO₂ decreases, equilibrium shifts left → fewer H⁺ → higher pH
CO₂ increases, equilibrium shifts right → more H⁺ → lower pH
Hyperventilation (↑ breathing rate):
CO₂ eliminated rapidly
Respiratory Alkalosis
Hypoventilation (↓ breathing rate)
CO₂ accumulates
Respiratory Acidosis
NH₃ freely diffuses, NH₄⁺ is trapped in urine and excreted
Generates new HCO₃⁻ for each H⁺ secreted
Reduces Hemoglobin's affinity for O₂ (facilitates delivery)
Bohr effect
Binds H⁺ directly
deoxygenated Hemoglobin binds more CO₂ and H⁺
Haldane Effect
excrete H⁺ in kidneys via NaH₂PO₄ (acidic salt)
fat metabolism, diabetic ketoacidosis
anaerobic exercise
carbonic acid dissociating into bicarbonate and hydrogen ions