jonka Rameen Sarwar 1 vuosi sitten
192
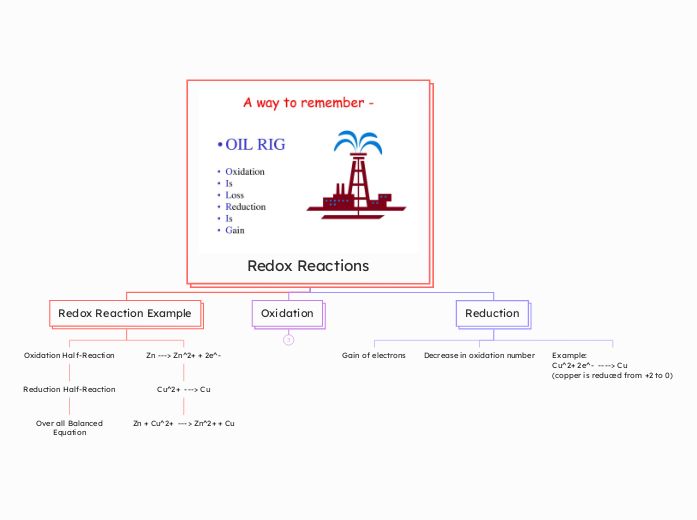
Redox Reactions
jonka Rameen Sarwar 1 vuosi sitten
192

Lisää tämän kaltaisia
Zn + Cu^2+ ---> Zn^2+ + Cu
Over all Balanced Equation