によって seyed Sajjadi 3年前.
414
Gas and Atmospheric Chemistry
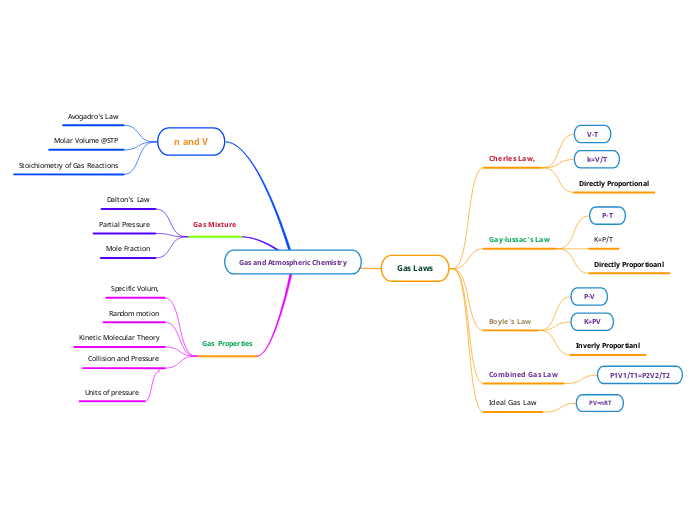
Gas and Atmospheric Chemistry
Gas Properties
Collision and Pressure
Units of pressure
Kinetic Molecular Theory
Random motion
Specific Volum,
Gas Mixture
Mole Fraction
Partial Pressure
Dalton's Law
n and V
Stoichiometry of Gas Reactions
Molar Volume @STP
Avogadro's Law
Gas Laws
Ideal Gas Law
PV=nRT
Combined Gas Law
P1V1/T1=P2V2/T2
Boyle's Law
Inverly Proportianl
K=PV
P-V
Gay-lussac's Law
Directly Proportioanl
K=P/T
P-T
Cherles Law,
Directly Proportional
k=V/T
V-T

