by Sourav Sharma 1 month ago
113
Science
by Sourav Sharma 1 month ago
113

Subtractive colour theory explains how pigments/inks absorb (subtract) certain wavelengths of light and reflect others — this is how we see colour in paint, printing, and objects.
Used In: Printing (printers, magazines) Painting Dyeing fabrics Color filters Primary Colours of Pigment: Cyan + Yellow + Magenta = Black (ideal) Colour Combinations (Subtractive Mixing): Cyan + Yellow = Green Magenta + Yellow = Red Cyan + Magenta = Blue All three = Black (in theory) — but usually a dark brown/gray, which is why printers use true black (K) Key Concept: The more pigment you add, the darker the color becomes. Pigments absorb light and reflect the color you see.
Additive colour theory explains how light creates colour. It’s based on combining different colours of light to form new ones.
Used In: TV screens Computer monitors Stage lighting Digital projectors Primary Colours of Light: Red + Green + Blue = White light Colour Combinations (Additive Mixing): Red + Green = Yellow Red + Blue = Magenta Green + Blue = Cyan All three (R + G + B) = White Key Concept: The more light you add, the lighter the color becomes. Adding all primary lights = white
The Human Eye Acts like a convex lens system. Focuses light onto the retina. Muscles adjust the shape of the eye's lens to focus on objects at different distances (accommodation). Vision problems are corrected with glasses or contacts (lenses!).
Flashlights & Headlights Use concave mirrors to focus and reflect light forward in a beam. Light source is placed at the focal point of the mirror to project a parallel beam.
Peepholes Use concave lenses to give a wide-angle view of people outside a door. The diverging lens makes the image smaller but lets you see a bigger area.
Microscopes Use multiple convex lenses to magnify very small objects. Objective lens produces a real, enlarged image, which is further magnified by the eyepiece (which creates a virtual image). Used in biology, chemistry, and medical labs.
Telescopes Combine convex lenses (or mirrors) to magnify distant objects. Two main types: Refracting telescope: uses lenses Reflecting telescope: uses mirrors Used in astronomy to study stars, planets, and galaxies.
Eyeglasses Concave lenses correct nearsightedness (myopia) by diverging light rays. Convex lenses correct farsightedness (hyperopia) by converging rays sooner. Designed based on how light needs to bend to reach the retina correctly.
. Cameras Use convex lenses to focus light on a sensor or film. Real, inverted image formed on the camera sensor. Zoom functions use lens combinations to adjust focus and magnification.
Magnifying Glass Uses a convex lens to enlarge small objects. Object is placed between F and O → virtual, upright, and larger image. Common in science labs and detective kits.
What Is a Concave Lens? A concave lens is thinner in the middle and thicker at the edges. It spreads out (diverges) light rays that pass through it. The rays appear to come from a virtual focal point in front of the lens. Light rays diverge after refraction The image is always virtual, upright, and smaller
Key Terms: Term Meaning Principal Axis Line that passes through the center of the lens Focal Point (F) Point where diverging rays appear to come from (virtual) Optical Center (O) Geometric center of the lens
Image Formation (SALT): Characteristic Description S (Size) Smaller than the object A (Attitude) Upright L (Location) Same side of the lens as the object T (Type) Always virtual (light rays don’t meet)
What Is a Convex Lens? A convex lens is thicker in the middle than at the edges. It bends (refracts) incoming light rays toward a common point called the focal point. Light converges (comes together) Can form real or virtual images, depending on object distance
Key Terms: Term Meaning Principal Axis Line through the center of the lens Focal Point (F) Where parallel rays meet after refraction Optical Center (O) Geometric center of the lens
Image Formation Based on Object Position: Object Position Image Characteristics (SALT) Type Beyond 2F (far from lens) Smaller, inverted, between F and 2F Real At 2F Same size, inverted, at 2F Real Between F and 2F Larger, inverted, beyond 2F Real At F No image (rays go parallel) N/A Between F and O Larger, upright, on same side of lens V
How to Identify SALT in Mirrors:
Convex Mirrors S: Smaller A: Upright L: Behind the mirror T: Virtual (Used in car side mirrors — “Objects in mirror are closer than they appear”)
What Is a Convex Mirror? A convex mirror is a curved mirror that bulges outward (like the back of a spoon). It diverges (spreads out) light rays, making them appear to come from a virtual focal point behind the mirror.
Key Characteristics: Property Description S (Size) Image is always smaller than the object A (Attitude) Image is always upright L (Location) Image appears behind the mirror T (Type) Image is always virtual (formed by backward tracing of rays)
How It Works: Incoming parallel rays reflect outward. When extended backward, they seem to come from a focal point behind the mirror. The image is always virtual, upright, and smaller.
Real-Life Uses: Security mirrors (stores, hallways) Side-view mirrors on cars (“Objects in mirror are closer than they appear”) ATMs and parking garages Blind spot mirrors for better visibility
Concave Mirrors SALT depends on the object's distance from the mirror: Far from mirror → Small, inverted, real image At focal point → No image (light rays are parallel) Closer than focal point → Larger, upright, virtual image
What Is a Concave Mirror? A concave mirror is a curved mirror that caves inward, like the inside of a spoon. It converges (brings together) light rays toward a single point called the focal point (F).
Key Parts of a Concave Mirror: Part Description Vertex (V) The center of the mirror surface Principal Axis A straight line passing through the center of the mirror Focal Point (F) The point where reflected rays converge (½ the distance to the center) Centre of Curvature (C) The center of the sphere the mirror is part of
Image Formation Depends on Object Location: Object Location Image Properties (SALT) Type Beyond C Smaller, Inverted, Between F & C Real At C Same size, Inverted, At C Real Between C and F Larger, Inverted, Beyond C Real At F No image (rays are parallel) N/A Between F and mirror Larger, Upright, Behind mirror Virtual
How It Works: Light rays reflect inward toward the focal point If rays actually meet = Real image If rays only appear to meet (when traced back) = Virtual image
Real-Life Uses: Makeup/shaving mirrors (close-up, virtual, enlarged) Satellite dishes (focus signals) Flashlights and headlights (focus light) Telescopes
Plane Mirrors S: Same size A: Upright L: Behind the mirror (same distance as the object is in front) T: Virtual (light rays don’t actually meet — it’s a visual projection)
What Is a Plane Mirror? A plane mirror is a flat, smooth reflective surface that reflects light according to the Law of Reflection (angle in = angle out). It forms clear, upright images that appear behind the mirror.
How It Works: Light rays bounce off the mirror following the Law of Reflection. The reflected rays appear to come from behind the mirror. Your brain traces those rays back, creating a virtual image.
Key Features: Reverses left and right (called lateral inversion) — e.g., text appears flipped Image is not real, meaning it cannot be projected onto a screen You see the image where the rays seem to come from, not where they actually bounce
What SALT Stands For: Letter Property What It Means S Size Is the image smaller, larger, or the same size as the object? A Attitude Is the image upright (right-side up) or inverted (upside down)? L Location Where is the image located compared to the object? (e.g., in front, behind, closer, farther) T Type Is the image real or virtual?
SALT is an acronym that helps describe any image formed by a mirror or lens:
Transmission
Light passes through transparent material
Glass window, air
Absorption
Light gets absorbed, turns to heat
Wearing black clothes in sun
Refraction
Index of Refraction (n)
What Is It? The index of refraction (n) is a number that tells you how much light slows down when entering a specific material from a vacuum (or air).
Where: n = index of refraction c = speed of light in a vacuum (3.00 × 10⁸ m/s) v = speed of light in the material The higher the value of n, the slower light moves in that material.
Common Refractive Indices: Material Approx. Index (n) Vacuum 1.00 Air 1.0003 Water 1.33 Glass 1.5 Diamond 2.42 Light bends more when going into materials with a higher n value.
Snell’s Law (Optional but useful) If you're applying math: N,1 , sin θ,1 = N,2 , Sin θ,2 n₁, n₂ = refractive indices of medium 1 and 2 θ₁, θ₂ = angles of incidence and refraction (measured from the normal) This tells you how much the light will bend!
Real-Life Examples: Eyeglass and camera lens design Optical fibers (total internal reflection) Prism color separation Diamonds sparkle because of high n!
Refraction of Light
What Is Refraction? Refraction is the bending of light when it passes from one material (medium) into another with a different optical density. Light slows down in denser materials Light speeds up in less dense materials This speed change causes the light ray to bend at the boundary between materials.
Why Refraction Happens: Light travels at different speeds in different media. When the speed changes, the direction changes (unless it hits at 90° to the surface). The more optically dense a material, the slower light travels in it.
Bending Rules: Situation Result Light enters a denser medium Bends toward the normal Light enters a less dense medium Bends away from the normal Light enters at 90° (normal) No bending — goes straight through Normal = an imaginary line perpendicular (90°) to the surface
Everyday Examples of Refraction: A straw looks bent in a glass of water Glass lenses bending light in eyeglasses Mirages on hot roads A pool that looks shallower than it really is
Light bends as it enters a new medium
From air into water or glass
Reflection
Law of Reflection
Types of Reflection:
Diffuse Reflection
Example: wall, paper, wood
No clear image formed
Light scatters in all directions
On rough surfaces
Specular Reflection
Example: flat mirror, calm lake
Produces clear images
On smooth surfaces
Key Properties of Reflection: Occurs on smooth, shiny surfaces (like mirrors or calm water) Angle in = Angle out (measured from the normal, not the surface) All rays, angles, and the normal lie in the same plane (flat geometry)
Definitions: Term What It Means Incident Ray The incoming ray of light hitting the surface Reflected Ray The ray bouncing off the surface Normal Line An imaginary line perpendicular (90°) to the surface Angle of Incidence (θi) Angle between the incident ray and the normal Angle of Reflection (θr) Angle between the reflected ray and the normal
The Law (Simple Rule): Angle of Incidence = Angle of Reflection
The Law of Reflection describes how light behaves when it hits a reflective surface (like a mirror). It tells us that light bounces off at the same angle it arrives — like a basketball hitting the floor at an angle and bouncing off evenly.
Light bounces off a surface
Mirror, still water
Key Assumptions of the Ray Model: Light travels in straight lines through a uniform medium. Light rays can reflect (bounce), refract (bend), or be absorbed. Light rays don’t curve unless they pass through or reflect off something.
The Ray Model of Light is a way to visualize how light travels. It treats light as a straight-line ray that moves in a specific direction from a source. This model helps us predict how light reflects, refracts, or gets absorbed when it hits different materials.
Why It’s Useful: Explains how images form in mirrors and lenses Helps with designing optical tools (telescopes, cameras, glasses) Supports laws like the Law of Reflection and Snell’s Law (for refraction)
Chemiluminescence
Light from a chemical reaction Example: glow sticks No heat involved (cool light)
Bioluminescence
Light produced by living organisms through chemical reactions Example: fireflies, deep-sea creatures
Phosphorescence
Similar to fluorescence, but glows after the light source is removed Example: glow-in-the-dark materials
Triboluminescence
Light from rubbing, crushing, or friction Example: Wint-O-Green Life Savers spark when crushed
Fluorescence
Light from phosphors absorbing UV rays Example: school ceiling lights Energy-efficient and bright
LED (Light Emitting Diode)
Light from electrical current flowing through a special material Efficient, long-lasting, low heat
Incandescence
Light from heat Example: filament in light bulbs Inefficient (produces more heat than light)
Natural light
other sources
Stars: Massive balls of gas that produce light like the Sun through nuclear fusion. Every visible star in the night sky emits natural light.
Bioluminescence: Light produced by living organisms through a chemical reaction inside their bodies.
Example: fireflies, jellyfish, anglerfish
Lightning: A powerful electrical discharge that emits a flash of light and heat in the sky.
Main Source
The sun
Drives photosynthesis in plants and controls day-night cycles on Earth.
Produces light through nuclear fusion—hydrogen atoms fuse into helium and release vast amounts of energy, including visible light, infrared, and UV radiation.
Natural light is any form of light that comes from a non-human source. These sources existed before technology and occur naturally in our environment. Most natural light is a result of nuclear or chemical reactions happening in nature.
Artificial light
Incandescent Bulbs:
A thin wire filament is heated by electricity until it glows. Produces light, but wastes a lot of energy as heat. Older and less efficient than newer technologies.
Lasers (Light Amplification by Stimulated Emission of Radiation):
Produce intense, focused beams of light at a single wavelength. Used in surgeries, CD/DVD readers, cutting tools, and military systems.
Neon Signs:
Glass tubes filled with gases like neon or argon that glow when electricity passes through. Common in advertising, art, and displays.
Fluorescent Lamps:
Use mercury vapor and a phosphor coating to emit UV light that is converted into visible light. More efficient than incandescent bulbs but can contain toxic substances.
LED Lights (Light Emitting Diodes):
Emit light when an electric current passes through a semiconductor. Very energy-efficient, long-lasting, and cool to touch. Used in screens, headlights, and modern household lighting.
Artificial light is created by humans using electrical or chemical technology. These sources are designed to function on demand and are widely used in homes, cities, electronics, and industries.
The wave model cannot explain all behaviors of light (like how it interacts with matter at small scales). For that, we use the particle model (photons) — more on that in quantum physics!
Why It Matters
Understanding wave properties is key for explaining how light, sound, and other energy forms behave. This knowledge helps in technology (Wi-Fi, medical imaging), music, communication, and even safety systems.
Types of Waves
Properties of Waves
Wavelength (λ): Distance between two consecutive crests or troughs (in meters). Frequency (f): Number of waves passing a point per second (in Hz). Amplitude: Height from the rest position to the crest or trough—relates to energy. Speed (v): How fast a wave travels. Formula: v = f × λ
Electromagnetic Waves
Electromagnetic Spectrum
Order of the Spectrum (From Longest to Shortest Wavelength)
Radio Waves Longest wavelength, lowest frequency Use: Communication (radio, TV, cell phones)
Microwaves Use: Cooking, radar, weather forecasting, satellite data
Infrared (IR) Felt as heat Use: Night vision, thermal imaging, remote controls
Visible Light Only part we can see Spectrum: ROYGBIV (Red has the longest λ, Violet the shortest)
Ultraviolet (UV) Higher energy than visible light Use: Sterilization, black lights, tanning Danger: Can cause skin damage
X-Rays Very short wavelength, high energy Use: Medical imaging, security screening
Gamma Rays Shortest wavelength, highest frequency and energy Use: Cancer treatment, nuclear reactions Can be dangerous in high doses
What Is It?
The electromagnetic (EM) spectrum is the complete range of all electromagnetic waves arranged by wavelength or frequency. All EM waves travel at the speed of light in a vacuum (3.00 × 10⁸ m/s), but they have different energies and uses based on their frequency and wavelength.
Do not need a medium — they can travel through space. Made of vibrating electric and magnetic fields. Examples: light, radio waves, X-rays.
Mechanical Waves
Require a medium to travel (like air, water, or solids). Examples: sound waves, water waves, seismic waves. Types: Transverse Waves: Particles move perpendicular to the wave direction (e.g., water waves). Longitudinal Waves: Particles move parallel to the wave direction (e.g., sound waves).
What Is a Wave?
A wave is a repeating disturbance or vibration that transfers energy from one place to another—without moving matter permanently. Waves can travel through solids, liquids, gases, or even empty space (in the case of light).
Why light can bend (refraction) Why it spreads around edges (diffraction) Why it shows interference patterns (wave overlapping)
Speed of Light (c):
Light travels at 3.00 × 10⁸ m/s in a vacuum. Formula: c = λ × f
Amplitude:
The height of the wave from its middle line (rest position). More amplitude = more energy (brightness).
Frequency (f):
How many waves pass a point in one second. Higher frequency = more energy. Unit: hertz (Hz)
Wavelength (λ):
The distance from one crest to the next. Shorter wavelengths = higher energy. Unit: nanometers (nm)
The Wave Model of Light explains light as a type of energy that travels in the form of waves. Like water waves or sound waves, light has measurable properties such as wavelength, frequency, and amplitude. This model helps explain many behaviors of light, including reflection, refraction, and diffraction.
breaks food into nutrients for energy and growth.
Respiratory System
Circulatory System
Nervous System
Skeletal System
Muscular System
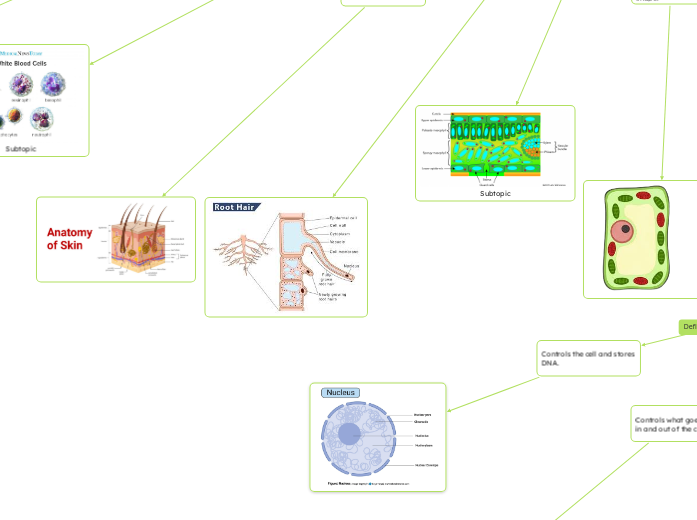
Integumentary System
Endocrine System
Excretory System
Reproductive System
Immune system
Lymphatic System
Interdependence
Respiratory & Circulatory: Lungs add oxygen → blood delivers it to cells. Digestive & Circulatory: Nutrients from digestion → absorbed into blood. Muscular & Skeletal: Muscles move bones → enable body movement. Nervous & Endocrine: Work together to control internal balance (homeostasis).
Lymph Nodes
Trap and destroy invaders.
Lymph Vessels
Transport lymph fluid
Returns fluid to blood, supports immune defense
Spleen
Helps produce and store immune cells.
Lymph Nodes
Filter harmful substances.
Antibodies
Proteins that target specific invaders.
white Blood cells
Fight pathogens.
Defends against infections and diseases
Male
testosterone (sperm ), genitals
Female
Ovaries (eggs), Uterus, Vagina
Produces sex cells and enables reproduction.
Urethra
Urine exits body.
Bladder
Stores urine.
Ureters
Carry urine to the bladder
Kidneys
Filter blood and produce urine.
Removes liquid Waster and maintains water balance
Adrenal Glands
Produce adrenaline and other stress hormones.
2
Regulates blood sugar (insulin/glucagon)
Thyroid Gland
Controls metabolism
Pituitary Gland
Master gland, Controls others
Produces hormones to control body processes.
Sweat Glands
Cool body through evaporation
Nails
Protect fingers and toes
Hair
Protects scalp; provides warmth
Skin
First barrier to environment; regulates heat.
Protects against infection, helps control body temperature
cardiac muscle
involuntary, only in heart.
smooth muscle
Involuntary, found in organs (e.g., intestines).
Skeletal muscle
Voluntary, moves bones.
works with bones to move body.
Cartilage
Cushions joints, reduces friction.
Bone marrow
Produces blood cells.
Joints
allow for movement between bones.
Bones
Provide structure and support
Supports body, protects organs, enables movement.
Neurons
Specialized cells that transmit impulses
nerves
Carry messages to muscle and organs.
spinal cord
Sends signals to and from the brain and body
brain
Main control center.
Sends electrical signals to control body actions.
blood
Made of red and White cells, plasma. platelets.
Capillaries
Exchange site between blood and cells.
veins
Carry blood back to the heart
Arteries
Carry blood away from the heart
heart
pumps blood through the body.
moves blood to deliver oxygen, nutrients,and remove waste
nose/mouth
Trachea (Windpipe)
passageway of air.
Bronchi/bronchioles
The tubes connected to the lungs
Lungs
contain alveoli for gas exchange
Alveoli
Tiny air sacs where O₂ enters blood,CO₂ exits.
Takes in O₂, removes CO₂ (gas exchange).
Pancreas
Releases digestive enzymes.
Liver
Produces bile to break down fats.
Large Intestine
Absorbs water; forms feces.
Small Intestine
Main site for nutrient absorption.
Stomach
Uses acid and enzymes to break down food.
Esophagus
Moves food to stomach by peristalsis.
mouth
Starts mechanical (chewing) and chemical (saliva) digestion.
Treatments
Surgery: Removes the tumor. Radiation: Destroys cancer cells with high-energy rays. Chemotherapy: Uses drugs to kill fast-dividing cells. Immunotherapy: Boosts the immune system to fight cancer.
Differences from Normal Cells
Stay in place
Can spread (metastasis)
Die if damaged
Keep dividing even if damaged
Controlled division
Uncontrolled division
What Causes Cancer?
how it works
Normal cells follow the cell cycle and stop dividing when necessary. Cancer cells ignore signals to stop dividing. They keep growing and may form a tumor (mass of abnormal cells). Malignant tumors can invade nearby tissues or spread (metastasize) to other parts of the body through blood or lymph.
Mutations (changes) in the DNA that control the cell cycle. Can be triggered by: Radiation (e.g., UV rays) Chemicals (carcinogens like tobacco smoke) Inherited genetic mutations Viruses (e.g., HPV)
A disease caused by uncontrolled cell growth and division, leading to the formation of tumors and potentially spreading to other parts of the body.
Maint Stages
Interphase
The longest phase, where the cell grows, carries out normal functions, and duplicates its DNA. G1 phase: Cell grows and performs its job. S phase: DNA replication occurs. G2 phase: Cell prepares for division by making proteins and organelles.
Mitosis
The cell divides its copied DNA into two identical sets. Includes prophase, metaphase, anaphase, and telophase.
Cytokinesis
The final step where the cell splits its cytoplasm, forming two separate daughter cells.
The series of stages a cell goes through to grow, prepare for division, and divide into two new cells.
Functions and Importance
Replace damaged or dead cells to maintain healthy tissue. Important for development, healing wounds, and in therapies like bone marrow transplants. Potential use in regenerative medicine and treating diseases.
Types of Stem Cells
Induced Pluripotent Stem Cells (iPSCs)
Adult cells genetically reprogrammed to act like embryonic stem cells.
Adult (Somatic) Stem Cells
Found in specific tissues (like bone marrow); mainly help repair and maintain those tissues.
Embryonic Stem Cells
Derived from early embryos; highly versatile.
Undifferentiated cells that can divide and develop into many different specialized cell types, playing a crucial role in growth, repair, and regeneration.
Characteristics
Potency
Totipotent: Can become any cell type including extra-embryonic (e.g., placenta). Pluripotent: Can become almost any cell in the body. Multipotent: Can become a limited range of cells within a tissue type.
Self-renewal: Can make copies of themselves over long periods without differentiating.
types of Animal Tissues
Connective tissue
Supports and connects body parts (e.g., bone, blood).
Nervous tissue
Sends and receives signals.
Epithelial tissue
Covers body surfaces and lines organs.
Muscle tissue
Contracts to create movement.
Types of Plant Tissues
Ground tissue
Provides support and stores nutrients.
Vascular tissue
Transports water and nutrients (xylem and phloem).
Dermal tissue
Protects the plant surface.
what are Tissues?
Collections of similar cells with the same function
Building blocks for organs.
Groups of specialized cells working together to perform a specific function.
Why its Important
Examples of Specialized cells
skin cells
Protect the body and reduce water loss.
Leaf cells (plants)
Does photosynthesis.
Root Hair cells (plants)
Absorb water and minerals.
White blood cells
Fight infections.
Red blood cells
Carry oxygen using hemoglobin.
Nerve cells (neurons)
Carry messages around the body.
muscle cells
Contract to allow movement.
Allows complex life and division of labor between cells.
Multicellular organisms need different cell types (e.g., muscle vs. nerve).
what is cell specialization?
process where cells develop specific structures/functions
Specialized cells = more efficient at one job
Happens during cell differentiation
organelles in both
Lysosomes
Breaks down waste and old cell parts.
Vacuole
Stores water, nutrients, and waste. Large in plants, small in animals.
golgi Apparatus
Packages and sends proteins and lipids.
endoplasmic Reticulum
Rough ER makes proteins; Smooth ER makes lipids and detoxifies.
Ribosomes
Makes proteins for the cell.
mitochondria
Produces energy (ATP) from glucose and oxygen.
cytoplasm
Jelly-like fluid where organelles float and reactions happen.
cell membrane
Controls what goes in and out of the cell.
nucleus
Controls the cell and stores DNA.
organelles in animals
centrioles
Help organize cell division.
lysosomes (mainly in animals)
Digest waste and invaders.
Organelles are specialized structures inside the cell that perform life-supporting tasks
organelles in plants
large central vacuole
Stores water and keeps plant firm.
chloroplasts
Makes food through photosynthesis using sunlight
cell wall
Rigid outer layer for support and shape.
The 6 Main Types of Reactions
Double Replacement Ions exchange between two compounds, forming new compounds. Example: AB + CD → AD + CB
Acid-Base (Neutralization) Acid reacts with a base to form a salt and water. Example: Acid + Base → Salt + H₂O
o
Acids and bases are two important types of chemical substances that have opposite properties and play vital roles in chemistry and everyday life. Acids are substances that release hydrogen ions (H⁺) when dissolved in water, making the solution acidic. Bases are substances that release hydroxide ions (OH⁻) in water, making the solution basic or alkaline. The interaction between acids and bases leads to many important chemical reactions.
The pH Scale
The pH scale is a measure used to indicate how acidic or basic a solution is, ranging from 0 to 14. Solutions with a pH below 7 are acidic, with stronger acids having values closer to 0. A pH of 7 is considered neutral, which is the pH of pure water. Solutions with a pH above 7 are basic or alkaline, with stronger bases approaching 14. The pH scale is logarithmic, so each whole number change represents a tenfold change in acidity or basicity.
Bases: Sodium hydroxide (NaOH), commonly called lye, used in soap making; calcium hydroxide (Ca(OH)₂), used in cement and water treatment.
Acids: Hydrochloric acid (HCl), found in stomach acid; sulfuric acid (H₂SO₄), used in car batteries and industry.
Neutralization Reaction
When acids and bases are mixed together, they react in a process called neutralization. During neutralization, the hydrogen ions from the acid combine with the hydroxide ions from the base to form water, while the remaining ions form a salt. This reaction is important in many applications, including medicine (antacids neutralizing stomach acid), agriculture (balancing soil pH), and industry (waste treatment). General formula: Acid + Base → Salt + Water
Properties of Bases
Bases have a bitter taste and a slippery, soapy feel when touched (like soap or baking soda). Unlike acids, bases usually do not react with metals to release hydrogen gas. Bases turn red litmus paper blue, indicating their basic nature. Their pH values are greater than 7, showing that they have a higher concentration of hydroxide ions than pure water.
Properties of Acids
Acids generally have a sour taste (like lemon juice or vinegar). They can react with certain metals, such as zinc or magnesium, to produce hydrogen gas. Acids turn blue litmus paper red, which is a common test used in laboratories to identify acidic substances. On the pH scale, acids have a value less than 7, meaning the concentration of hydrogen ions is high compared to pure water.
Combustion A substance reacts with oxygen, releasing energy (often producing CO₂ and H₂O). Example: Hydrocarbon + O₂ → CO₂ + H₂O
Single Replacement One element replaces another element in a compound. Example: A + BC → AC + B
Decomposition A compound breaks down into two or more simpler substances. Example: AB → A + B
Synthesis (Combination) Two or more simple substances combine to make a more complex compound. Example: A + B → AB
Chemical reactions can be classified into different types based on how substances change during the reaction.
Balancing Chemical Equations
Why Balance Equations? Obeys the Law of Conservation of Mass (mass can’t be created or destroyed) Ensures equal atoms on reactants and products side Shows correct mole ratios for calculations in chemistry
Write the unbalanced equation with correct formulas. Count atoms of each element on both sides. Use coefficients (numbers in front) to balance atoms (never change subscripts). Start balancing with elements that appear once on each side. Balance hydrogen and oxygen last (if present). Double-check atom count on both sides after adjusting. Make sure all coefficients are in the lowest possible ratio (simplify if needed).
Balancing a chemical equation means making sure the number of atoms of each element is the same on both sides of the reaction.
Chemical Equations (Symbolic Format)
A chemical equation shows the actual chemical formulas of all substances involved. It provides specific, measurable information about the atoms and molecules taking part in the reaction.
Parts of a Chemical Equation: Component What It Means Reactants Starting materials (left of arrow) Products New substances formed (right of arrow) Arrow (→) "Yields" or "produces" Coefficients Numbers in front of formulas (affect entire molecule) Subscripts Small numbers within formulas (show atoms per molecule)
Format: H₂ + O₂ → H₂O (Unbalanced) Balanced Form (to follow the Law of Conservation of Mass): 2H₂ + O₂ → 2H₂O Why Balancing Matters: Ensures the same number of atoms on both sides Obeys physical laws (mass is conserved) Shows correct mole ratios used in stoichiometry
Format: Reactant + Reactant → Product(s) Example: Hydrogen + Oxygen → Water Good for: Introducing reactions to beginners Understanding the general change Simple verbal communication Limitations: Doesn’t show actual elements or compounds Doesn’t help with balancing or atom counting Can’t see ratios or formul
A word equation shows the names of the substances involved in a chemical reaction. It gives you a basic understanding of what's reacting and what's being formed, without showing the actual chemical formulas.
Counting atoms means figuring out how many of each type of atom is present in a chemical formula.
Look at the subscript → the small number written after an element. Ex: In H₂O → There are 2 hydrogen atoms and 1 oxygen. If a subscript is outside brackets, it applies to everything inside. Ex: Ca(OH)₂ → 1 calcium, 2 oxygen, 2 hydrogen. Coefficients (numbers in front) apply to everything in the formula. Ex: 3H₂O → 3 × 2 = 6 H and 3 × 1 = 3 O
No subscript? → Just 1 atom. Always multiply subscripts and coefficients when both are present. Polyatomic ions in brackets must be counted carefully.
Made of two or more non-metals. Electrons are shared, not transferred (unlike ionic compounds). Form covalent bonds. Typically exist as discrete molecules (e.g., H₂O, CO₂).
Only non-metals → use prefix naming system. Use a periodic table to check if both elements are non-metals (right side). Practice makes perfect — these names can feel tricky at first.
Naming Rules for Molecular Compounds Use prefixes to show how many atoms of each element are present. The second element ends in -ide. Prefixes: Number Prefix 1 mono- 2 di- 3 tri- 4 tetra- 5 penta- 6 hexa- 7 hepta- 8 octa- 9 nona- 10 deca- Don’t use “mono-” on the first element if there’s only one.
Key Properties Low melting and boiling points (many are gases or liquids). Do not conduct electricity in solid or liquid state. Weaker forces between molecules compared to ionic solids.
Polyatomic Ions
Polyatomic ions are groups of atoms bonded together that act as a single ion. Even though they contain multiple elements, they carry one overall charge and move as a unit in reactions. These ions don’t break apart when forming compounds.
Naming Rules 1. Do Not Change the Name Unlike non-metal endings that change to “-ide” (like “chloride”), polyatomic ions keep their names. 2. Use Brackets When Needed If you need more than one polyatomic ion, put the entire ion in brackets before adding a subscript.
Transition Metals (Multivalent Metals)
Transition metals are elements found in the middle block of the periodic table (Groups 3–12). They’re called “multivalent” because they can form more than one kind of positive ion (cation) — meaning, they can lose different numbers of electrons.
Naming Rule: Use Roman numerals to show the metal’s charge in the name Examples: FeCl₂ → Iron (II) Chloride (Fe²⁺) FeCl₃ → Iron (III) Chloride (Fe³⁺) CuO → Copper (II) Oxide
Simple Ionic Compounds
Metal (single charge) + Non-metal Metal forms positive ion (cation) Non-metal forms negative ion (anion) Combine to balance charges
Naming Rule: Metal name + non-metal (ending → “-ide”) No prefixes Examples: NaCl → Sodium Chloride CaF₂ → Calcium Fluoride Li₂O → Lithium Oxide
Common Signs of a Chemical Reaction
Irreversibility
The reaction can't easily be reversed (e.g., cooking an egg)
y
Change in Odor
A new smell can appear (e.g., rotting eggs smell due to sulfur compounds)
Formation of Percipitate
A solid forms from two liquids (e.g., milk + acid = curds)
Light production
Some reactions emit light (e.g., fireworks)
Temperature Change
Heat is released (exothermic) or absorbed (endothermic)
Gas production
Bubbling, fizzing, or a new odor indicates a gas is released
Colour Change
A new color appears (e.g., copper turns green)
A chemical reaction occurs when substances combine or break apart to form new substances with different properties.
What is a Bohr Diagram
A simplified way to represent an atom’s structure. Shows the nucleus (with protons and neutrons) and electrons in energy levels (shells). Developed by Niels Bohr.
Visual models that show how electrons are arranged around the nucleus.
Law of Conservation of Mass
What It Means: The number of each type of atom stays the same before and after a reaction. Chemical reactions only rearrange atoms, they don’t add or remove any. You should always be able to account for every atom.
Helps us balance chemical equations properly. Explains why atoms and mass must be counted carefully. Supports that all chemical changes must follow this rule.
Real-Life Examples: Burning wood: The ashes may seem lighter, but gases like CO₂ and H₂O escape — total mass remains the same. Sealed flask reactions: No matter how violent or colourful the reaction, the total mass stays constant if it’s closed.
The Law of Conservation of Mass states that mass is neither created nor destroyed in a chemical reaction. The total mass of reactants = total mass of products.
What is an Atom
Ions
Atoms can gain/lose electrons: Cation (+) → loses electrons (more protons than electrons) Anion (–) → gains electrons (more electrons than protons)
Isotopes
Same number of protons, different number of neutrons Same element, different atomic mass Example: Carbon-12 (6p⁺, 6n⁰) Carbon-14 (6p⁺, 8n⁰)
Atomic Number And Mass number
Mass Numbers
The total number of protons + neutrons in the nucleus. Mass number is not on the periodic table — it’s often rounded from atomic mass. Used to calculate the number of neutrons: Neutrons = Mass Number – Atomic Number Example: Carbon-12 Atomic Number = 6 (protons) Mass Number = 12 Neutrons = 12 – 6 = 6
Atomic Numbers
The number of protons in the nucleus of an atom. Defines the element — each element has a unique atomic number. Example: Carbon has 6 protons → Atomic Number = 6 In a neutral atom, the number of electrons = protons.
Subatomic Particles
Particle Symbol Charge Location Relative Mass Notes Proton p⁺ +1 Inside nucleus 1 Determines the element’s identity Neutron n⁰ 0 Inside nucleus 1 Adds mass, helps stabilize nucleus Electron e–1 Outside in shells ~0 Involved in bonding and reactions
Definition: The basic unit of matter; everything around us is made of atoms. Atoms cannot be broken down into simpler substances by chemical means. Made of protons, neutrons, and electrons.
Trends
Reactivity – How easily elements react Electronegativity – Attraction for electrons Conductivity – Ability to conduct electricity
other key information about the periodic table
Valence Electrons
Electrons in the outermost shell Determine how an atom will bond or react Group 1 = 1 valence electron Group 17 = 7 valence electrons
Atomic mass
Average mass of protons + neutrons Used to calculate isotopes and molar mass
Atomic numbers
Number of protons in an atom Determines the element’s identity Example: Atomic number 6 = Carbon
Metals, Non-Metals, and Metalloids
Metalloids (border staircase)
Dull, brittle (if solid), poor conductors Tend to gain electrons (form negative ions) Can be gases, liquids, or solids 🧪 Examples: Oxygen, Sulfur, Nitrogen, Carbon
Non-Metals (right side of staircase)
Properties of both metals and non-metals Semi-conductors (used in electronics) 🧪 Examples: Silicon (Si), Boron (B), Arsenic (As)
Metals (left side of staircase)
Shiny (lustrous), malleable, ductile Good conductors of heat and electricity Tend to lose electrons (form positive ions) Examples: Iron, Gold, Aluminum, Copper
Group 1: Alkali Metals
Very reactive, especially with water Soft, shiny metals Reactivity increases down the group Stored in oil (to prevent explosions with air/water) Examples: Lithium (Li), Sodium (Na), Potassium (K) Reacts explosively with water
Group 2: Alkaline earths metals
Also reactive, but less than Group 1 Burn with bright flame colors Found in the Earth’s crust (limestone, bones, shells) Examples: Magnesium (Mg), Calcium (Ca), Barium (Ba)
Groups 3-12: Transition metals
Multivalent: Can form ions with different charges Often form colored compounds Good conductors of heat and electricity Used in coins, jewelry, wiring, construction Examples: Iron (Fe²⁺/Fe³⁺), Copper (Cu⁺/Cu²⁺), Zinc (Zn²⁺)
Group 17: Halogens
Very reactive non-metals Need 1 more electron to fill outer shell Often form salts with metals (e.g., NaCl) Examples: Fluorine (F), Chlorine (Cl), Iodine (I) Toxic and corrosive in elemental form
Group 18: Noble Gases
Very stable and unreactive (inert) Full outer electron shell (8 valence electrons) Used in lighting, signs, and as non-reactive atmospheres Examples: Helium (He), Neon (Ne), Argon (Ar)
Columns = Groups/Familles (1 to 18)
Elements in the same group
react in similar ways
Show similar chemical properties
Have the same number of valence electrons
How the Table Is Arranged Rows = Periods (1 to 7) Show how many electron shells (energy levels) an atom has Example: Period 2 → 2 electron shells Properties gradually change as you move left to right: Metallic → Non-metallic Reactive → Stable
A Chart organizing all known chemical elements based on their atomic number, electron configuration, and recurring chemical properties
Invented by: Dmitri Mendeleev (1869) Modern version: Arranged by increasing atomic number (number of protons) 118 elements currently (as of 2025)
Chemical Properites
Flammability Reactivity with water or acids Ability to rust or tarnish
Physical Properties
Colour Texture Odour Melting/Boiling Point Density Solubility Conductivity
Heterogeneous mixtures
Multiple visible parts or phases
Non-uniform composition
Can be separated by physical means
Two or more substances physically combined
Homogeneous Mixtures (solutions)
one visible phase
Uniform composition
Compounds
Fixed ratio of elements
two or more elements chemically bonded
Elements
Found on the periodic table
only one type of atom
Pure Substances
Has constant composition and properties
Made up of one type of particle
Signs of Chemical Change
Temperature change
Light or sound produced
Precipitate formation (solid from liquids)
Gas formation
colour change
Chemical change
Rusting, burning, baking a cake
often irreverisble.
A new substance with new properties is created.
physical change
Melting ice, dissolving salt, breaking glass
Easily reversible
Change in form or State, but no new substance created
Gas
high energy
Easily compressible
Particles move freely and quickly
No fixed shape or volume
Liquid
Subtopic
Moderate energy
Slightly compressible
Particles slide past eachother
No fixed shape but fixed volume
Solid
Low energy
Not compressible
Particles are tightly packed and vibrate in place
Fixed shape and volume
Particles move faster when heated and slower cooled.
Particles are attracted to each other.
Particles are always moving.
Particles have space between them.
All matter is made of tiny particles.