przez Merhamah Khan 6 lat temu
3573
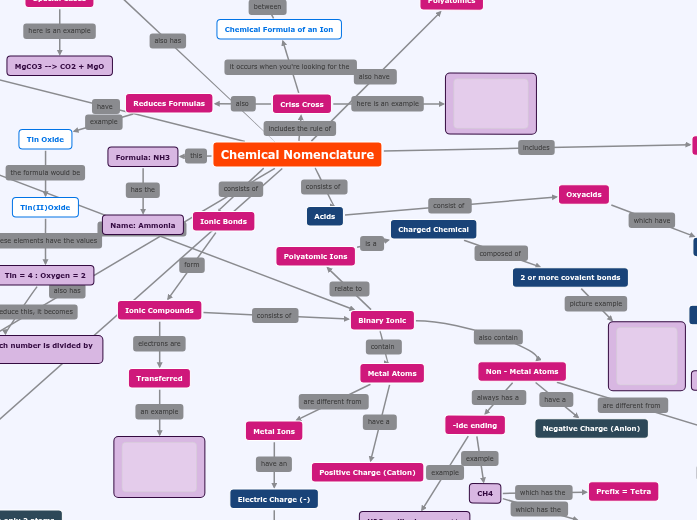
Chemical Nomenclature Concept Map
przez Merhamah Khan 6 lat temu
3573

Więcej takich
CO3 2- (on top)
Tin(II)Oxide
Tin = 4 : Oxygen = 2
Tin (II)Oxide --> each number is divided by 2
-ous endings
Nitric acid or Nitrous acid
Polyatomic Ions
Charged Chemical
2 or more covalent bonds
Non - Metal Atoms
Non - Metal Ions
Negative Charge
H- = hydride
Negative Charge (Anion)
-ide ending
CH4
Suffix = hydride
Prefix = Tetra
H2O = dihydrogen oxide
Water (common name)
Metal Atoms
Metal Ions
Electric Charge (-)
Positive Charge (Cation)