作者:THOMAS JENKINS 3 年以前
300
Atmospheric gases
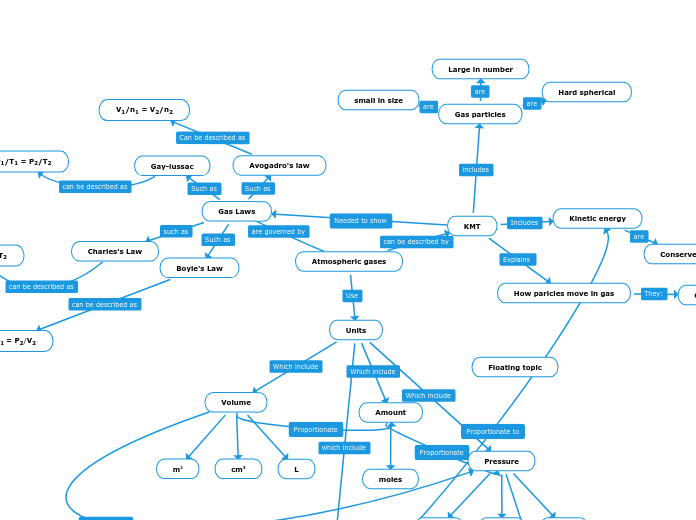
Floating topic
Ideal Gas law
STP
SATP
Molar gas volume
Atmospheric gases
KMT
Gas particles
Hard spherical
Large in number
small in size
Kinetic energy
Conserved
How paricles move in gas
Continue until collision
Gas Laws
Avogadro's law
V1/n1 = V2/n2
Gay-lussac
P1/T1 = P2/T2
Charles's Law
V1/T1 = V2/T2
Boyle's Law
P1/V1 = P2/V2
Units
Amount
moles
Pressure
kPa
Pa
bar
atm
Temperature
K
C
Volume
L
cm3
m3

