作者:Kennedy Byars 4 年以前
426
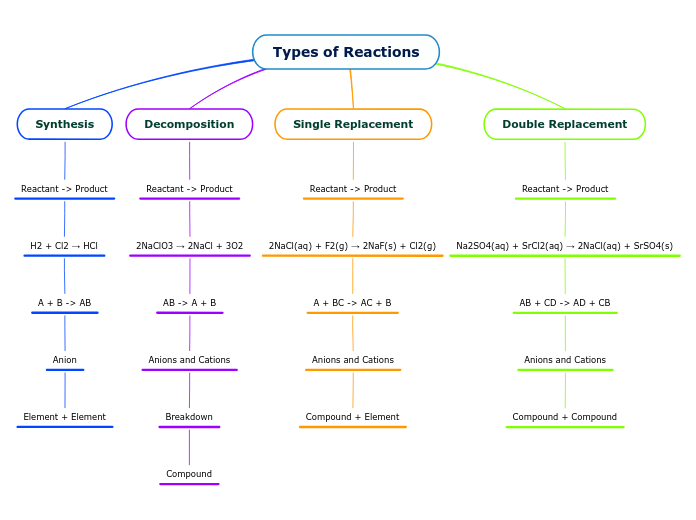
Types of Reactions
作者:Kennedy Byars 4 年以前
426

更多类似内容
AB + CD -> AD + CB
Compound + Compound
A + BC -> AC + B
Compound + Element
AB -> A + B
Anions and Cations
Breakdown
Compound
A + B -> AB
Anion
Element + Element