by Siti Zahrah 6 years ago
2345
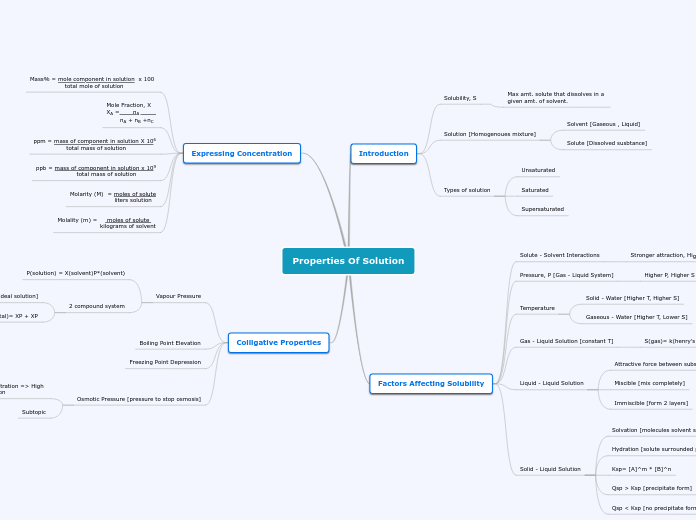
Properties Of Solution
by Siti Zahrah 6 years ago
2345

More like this
P(total)= XP + XP
Raoult's Law [ideal solution]